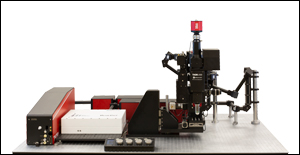
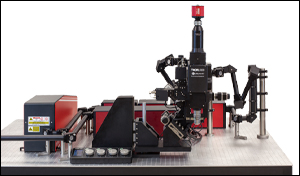
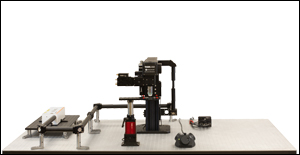
多光子顕微鏡 Bergamo® IIシリーズ

Please Wait
多光子顕微鏡 Bergamo® IIシリーズ
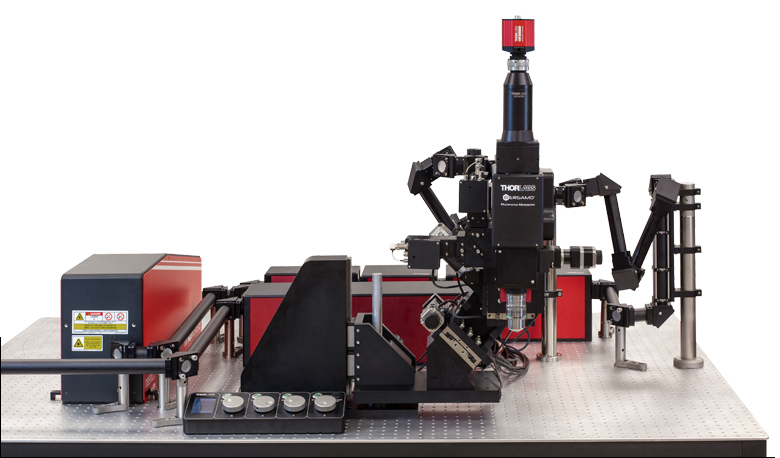
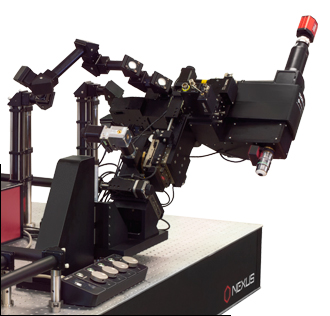
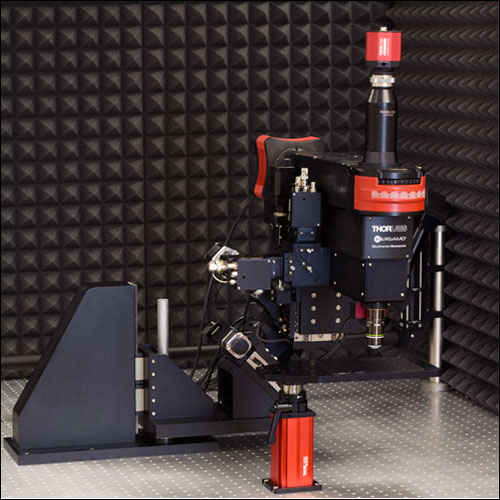
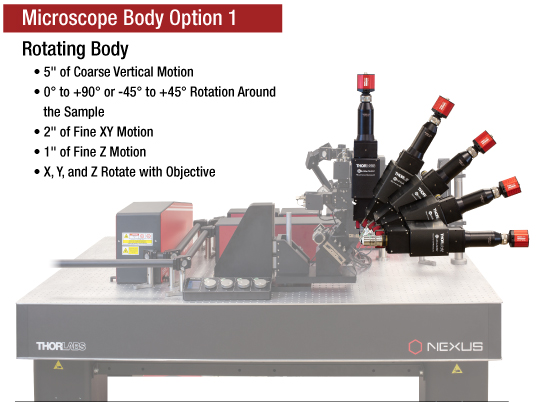
当社では試料を顕微鏡に適合させるのではなく、顕微鏡を試料に適合させるべきであるという方針に基づき、様々な実験要件に対応可能なモジュール式の多光子イメージングプラットフォームを開発しました。当社の多光子イメージングシステムは、微小電極などを用いた従来の研究手法に比べて、高速、高深度、高強度でニューロン集団の同時読み出しと操作が可能です。 下のオプション一覧および右下の回転式Bergamo顕微鏡の5軸移動の動画でご紹介しているように、当社では回転式ボディを開発し、これにより顕微鏡が試料の周りを回転できるようになりました。当社では固定式ボディのタイプもご用意しています。このタイプではアーム下の奥行きが196.5 mmあり、対物レンズ周りに大きな作業スペースを3次元的に確保できます。
「ハイライト」、「特長」および「モジュール」タブでご紹介しているBergamo IIの特長には、操作性を損なうことなく最先端の性能を得ることに注力してきたことが反映されています。Bergamo顕微鏡の推奨用途についての詳しい説明は「用途」タブに記載されています。また、独自の顕微鏡を設計されるお客様向けに、「構成」タブでは主な特長と推奨する用途を記した多数の構成例を掲載しています。このプラットフォームは実験のニーズに応じて簡単に変更やアップグレードができます。対応可能なアドオンおよびアップグレードについては「レトロフィット」タブをご覧ください。
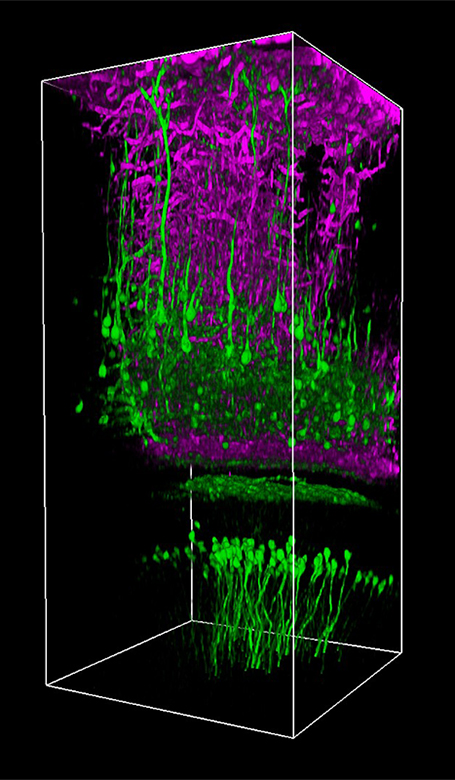
Bergamoイメージングシステムを使用することで、優れた試料イメージ(「イメージングギャラリ」タブ参照)を得ることができ、それらは数多くの出版物に掲載されています(「関連出版物」タブ参照)。 右下の「共同研究で生まれたイノベーション」の動画でご覧いただけるように、当社のライフサイエンス部門はUniversity College LondonのDr. Michael Hausserの研究グループと連携し、彼らのニーズに合ったカスタム仕様のBergamo顕微鏡システムを設計してきました。当社のBergamoイメージングシステムについてのご質問は、ボックス内のContact USボタンをクリックして当社までお問合せください。
Five-Axis Movement of Rotating Bergamo Microscopes |
ハイライト
ベッセルビームを用いた高速ボリューム画像取得
当社ではHoward Hughes Medical Institute(HHMI)とNa Ji教授(カリフォルニア大学バークレー校)の協力を得て、Bergamo®多光子レーザ走査型顕微鏡用のベッセルビームモジュールをご提供しています。 神経細胞活動のin vivoボリュームイメージングを行うには、サブミクロンの空間分解能とミリ秒の時間分解能の両方が必要です。従来の方法では、回折限界のガウシアンビームを走査して3次元画像を作成しますが、ベッセルビームを用いた多光子イメージングでは、軸方向に細長い集光スポットを利用してボリューム画像を取得します。励起光の被写界深度が拡大されたことにより3次元の体積が2次元に投影され、2次元フレームレートは効率的に3次元ボリュームレートに変換されます。
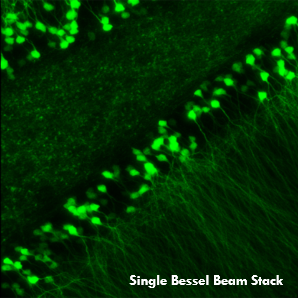
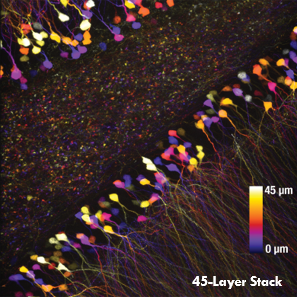
Ji氏の先駆的な研究で実証されたように、このベッセルビームを用いた高速のイメージング技術はシナプス解像度を有し、Ca2+ダイナミクスや、マウスとフェレットの視覚皮質における樹状突起スパインの調整特性などの計測ができます。 下の画像ではこのベッセルビームを用いた多光子イメージング技術の能力を示しています。Thy1-GFP-Mマウスの脳切片の300 x 300 μmの範囲を、それぞれベッセルビーム(左)およびガウシアンビーム(右)で走査した画像を比較しています。ガウシアンビームを集光して取得された45枚の光学的なスライス画像を垂直に積み重ねるとボリュームイメージが得られますが、長さ45 μmの集光スポットを有するベッセルビームを使用すると、1回走査するだけで同じ構造的な特徴を見ることができます。これはボリュームイメージングの速度が大幅に向上することを示しており、この手法はin-vivoでまばらにラベル付けされた試料を調べるのに適しています。
お持ちのBergamo顕微鏡にベッセルビームによるイメージング機能の追加を希望される場合は、当社までご連絡ください。ご提供可能なアップグレードとアドオンについては、「レトロフィット」のタブをご覧ください。
出典: Lu R, Sun W, Liang Y, Kerlin A, Bierfeld J, Seelig JD, Wilson DE, Scholl B, Mohar B, Tanimoto M, Koyama M, Fitzpatrick D, Orger MB, and Ji N. "Video-rate volumetric functional imaging of the brain at synaptic resolution." Nature Neuroscience. 2017 Feb 27; 20: 620-628.
1回のベッセルビームによる走査画像(左)は、ガウシアンビームによる45枚の光学画像の断片を積み重ねて得られた体積走査画像(右)と同じ構造情報を捉えており、これは全走査時間を45分の1に短縮できることを示しています。これらの画像は脳切片の300 μm x 300 μmの範囲を走査したものです。ガウシアンビームによる画像を積み重ねた画像の深さはスケールバーで示されています。試料ご提供:Qinrong Zhang, PhD and Matthew Jacobs; the Ji Lab, Department of Physics, University of California, Berkeley.
Click to Enlarge
Thy1-YFPマウス(オス、21週)の画像。1300 nm、繰り返し周波数326 kHz、パルス幅約60 fsで取得。観察窓の中心は、体性感覚皮質上部、ブレグマから横方向に2.5 mm、後方に2 mmの位置にあります(皮質表面でのレーザーパワーは1.1 mW)。画像ご提供:Chris Xu Group, Cornell University.
3光子イメージング
当社では、Bergamo多光子顕微鏡に3光子イメージング技術を導入できるように、800~1800 nmの波長範囲に対応する走査光路用の光学素子を開発しました。3光子励起は深部組織イメージングに適していますが、一般には波長1300 nmまたは1700 nm付近の高パルスエネルギーの励起光源が必要です。2光子イメージングと比較して、3光子イメージングでは組織による散乱が少なく、また焦点の合わないバックグラウンド光が抑制されるので、信号対バックグラウンドの比が改善されます。
下の写真のように、3光子イメージングが可能なシステムの構成要素として、2光子と3光子の同時イメージングをサポートするダイクロイックミラーや、広帯域でのサンプリングができる繰り返し周波数の低いレーザをサポートするエレクトロニクスを含めることができます。ThorImage®LSソフトウェアでは、3光子検出のための重要な機能を強化しています。例えば、3光子信号の検出を励起パルスと同期させ、最良の信号対雑音比が得られるように位相遅延を制御することができます。詳細については「ThorImageLS」タブをご参照ください。
お持ちのBergamo顕微鏡に3光子イメージング機能の追加を希望される場合は、当社までご連絡ください。ご提供可能なアップグレードとアドオンについては、「レトロフィット」タブをご覧ください。
出典: Wang T and Xu C. "Three-photon neuronal imaging in deep mouse brain." Optica. 2020; 7 (8): 947-960.
Click to Enlarge
2光子および3光子イメージングの構成。詳細については「構成」のタブをご参照ください。
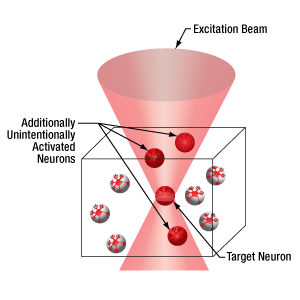
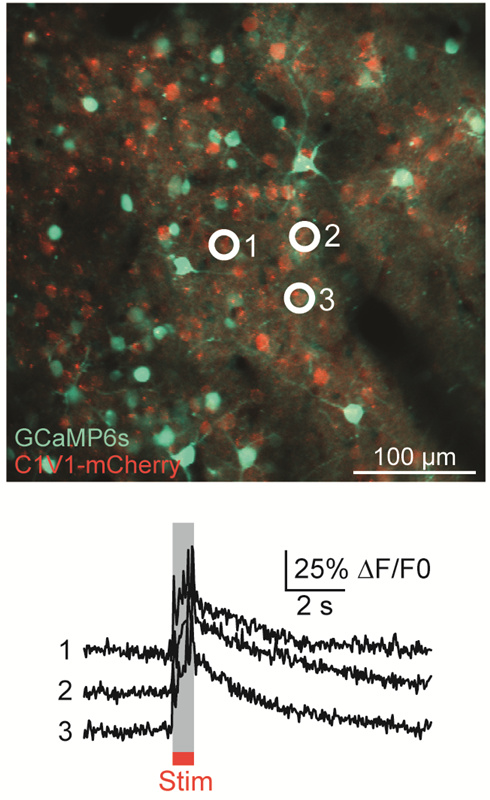
空間光変調器(SLM)を用いた同時多点光刺激
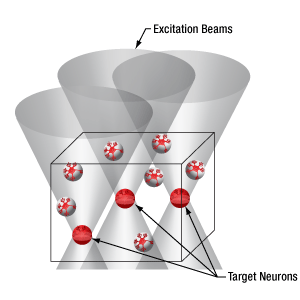
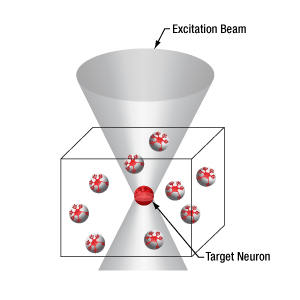
当社の空間光変調器(SLM)を用いると、ホログラフィックパターンにより試料内の複数の位置の光刺激を同時に行うことができます。この空間光変調器はフェムト秒パルスレーザによる2光子励起用に設計されており、光刺激用レーザのビームプロファイル全体に渡って位相を操作し、ユーザが決定した数百の焦点を生成することができます。
下の図では、空間光変調器を使用した2光子刺激の利点を、通常の2光子刺激や単一光子による光刺激と比較して示しています。単一光子による光刺激では、1つの細胞だけをターゲットにすることができず、ターゲットとする細胞に近接する細胞も刺激してしまいます。この問題は、1つの細胞をターゲットにできる分解能を有する2光子刺激を利用することで解決します。しかし、その場合でも同時にターゲットにできる細胞は1つに制限されます。2光子刺激と空間光変調器を組み合わせると、多数の焦点を生成して複数の細胞を同時に刺激することができるため、その制限を克服することができます。刺激用ビームの形状は光刺激の効果が向上するように成形することができます。これは、単一視野内の異なる深度にある神経集団を活性化させるために重要な機能です。 空間光変調器の位相マスクのパターンは素早く切り替えることができ、複数の独立した焦点を任意のシーケンスで個々のターゲットに向けることができます。校正プロセス、ホログラムの生成、外部ハードウェアとの同期などは、すべてThorImage®ソフトウェアで管理され、シームレスな制御が可能です。 詳細については「ThorImageLS」タブをご覧ください。
お持ちのBergamo顕微鏡に空間光変調器(SLM)によるイメージング機能の追加を希望される場合は、当社までご連絡ください。ご提供可能なアップグレードとアドオンについては、「レトロフィット」タブをご覧ください。
2光子刺激+空間光変調器(右)による方法では、複数のターゲット細胞を同時に刺激することが可能です。これは、単一光子(左)や2光子(中央)で刺激する一般的な方法では不可能です。
特長
Bergamo®IIシリーズ顕微鏡の下記の多くの機能は、既存の当社顕微鏡システムにも追加できます。詳細については「レトロフィット」のタブをご参照ください。
| レーザ走査、ワイドフィールド観察および撮像、透過照明イメージング | ||
|---|---|---|
| 走査 | レゾナント-ガルバノ-ガルバノ |
|
| ガルバノ-レゾナント |
| |
| ガルバノ-ガルバノ |
| |
| 空間変調モジュール(SLM) |
| |
| ワイドフィールド観察および撮像 |
| |
| 落射照明 | ||
| Dodt勾配コントラストならびにDICモジュール |
| |
| 光学性能 | ||
|---|---|---|
| PMT構成 |
| |
| 対物レンズと1枚目の集光レンズの間の距離の最小化 |
| |
| ペリスコープ |
| |
| 自社設計の走査光路 |
| |
| 操作性 | ||
|---|---|---|
| 数種類のソフトウェアパッケージ |
| |
| タッチスクリーン式コントローラ |
| |
| アクセスが簡単な吸収フィルタとダイクロイックホルダ |
| |
| 入出力トリガ |
| |
| 対物レンズ下の大きな調整可能な空間 |
| |
焦点面が≥90°回転 |
| |
| ビーム調整モジュール | ||
|---|---|---|
| 高速光変調器 |
| |
| ポッケルスセル |
| |
| 可変減衰器 |
| |
| ビーム安定化装置 |
| |
| ベッセルビームを使用したボリュームイメージング |
| |
| サンプルホルダ | ||
|---|---|---|
| スライド、記録チャンバ、またはプラットフォーム用高剛性スタンド |
| |
| マイクロマニピュレータ用XYプラットフォーム |
| |
| 当社サポート | ||
|---|---|---|
| すべて自社設計、自社製造 |
| |
| モジュール式システム構成 |
| |
| 当社技術者による組み立て/設置 |
| |
| クイックサポート |
| |
当社では、用途に応じて様々なご要望にお応えできるように、
お客様のニーズに合わせたご提案を心掛けています。
ご意見・ご要望、またご質問などございましたら当社までお気軽にご連絡ください。
Bergamo® IIシリーズモジュール
当社のBergamo® II顕微鏡は、実験の要件に合わせ構成をカスタマイズ可能なモジュール式システムです。下記のモジュールは、お客様のご検討用に様々なシステム構成例をご紹介した「構成」のタブで紹介されています。
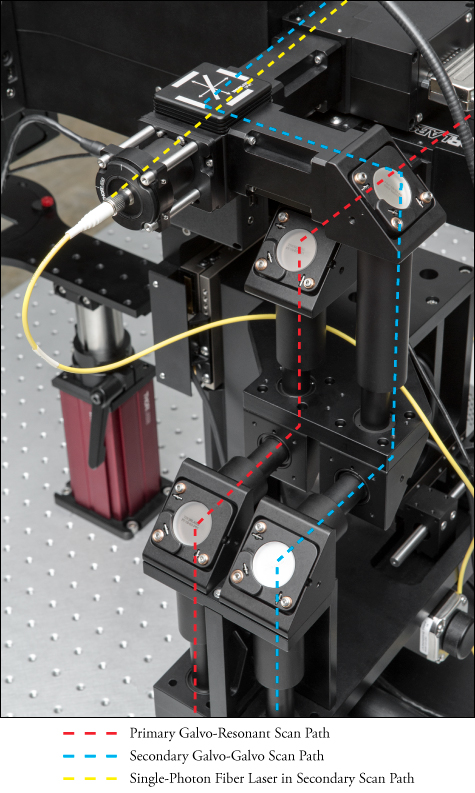
ガルバノ-レゾナントスキャナ、ガルバノ-ガルバノスキャナ、空間変調モジュール(SLM)
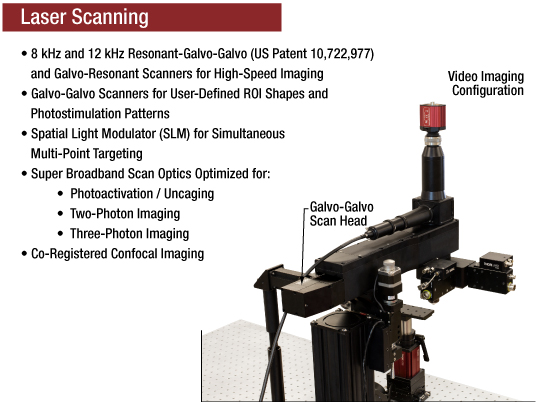
Bergamo® II顕微鏡は、入射ビームを伝搬、調整、そして誘導する1つもしくは2つの走査光路で構成することができます。各走査路では、レゾナント-ガルバノ-ガルバノスキャナ、ガルバノ-レゾナントスキャナ、ガルバノ-ガルバノスキャナ、または空間変調モジュール(SLM)をご使用いただけます。これらの選択肢により、高フレームレート、高感度、さらには関心領域(ROI)へのターゲット照射など、各実験要件に合わせて走査系を最適化することができます。
ランダムアクセス走査向けレゾナント-ガルバノ-ガルバノスキャナ
当社では8 kHzならびに12 kHzのレゾナント-ガルバノ-ガルバノスキャナ(RGG、US Patent 10,722,977)をご用意しております。これらのスキャナにより、単一視野内の複数の領域を高速かつ立て続けにイメージングすることが可能となります。8 kHzスキャナは視野全体を使用し、最大フレームレートは400 fpsです。一方、12 kHzスキャナのフレームレートは600 fpsにまで増加します。
高速イメージング用ガルバノ-レゾナントスキャナ
当社では8 kHzならびに12 kHzのガルバノ-レゾナントスキャナをご用意しております。8 kHzスキャナは視野全体を使用し、最大フレームレートは400 fpsです。一方、12 kHzスキャナのフレームレートは600 fpsにまで増加します。
お客様が指定する関心領域(ROI)形状に対応するガルバノ-ガルバノスキャナ
ガルバノ-ガルバノスキャナは、お客様が描いた走査形状(線状、ポリライン、正方形、長方形)ならびに光刺激のカスタムパターン(円、楕円、多角形、点)などに対応します。ピクセル滞在時間に一貫性があり、信号をより良く積分し、イメージング画像が均一化されます。
同時多点光刺激に適応した空間変調モジュール(SLM)
物理的に点から点へ移動するスキャナとは異なり、空間変調モジュール(SLM)は、ホログラフィを用いてビームを回折させ、ユーザが望むパターンに成形します。装置は一般的なビーム成形と、視野範囲での多数の集光点生成に対応します。後者ではこれにより試料内の複数の箇所を同時に光励起することができます。
Click to Enlarge
図2. こちらの合成画像では、最適な励起波長750 nmと835 nmの高速切り替えにより高コントラストが得られています。2つのチャンネルセットは7 fpsのイメージング速度で取得しました。
Click to Enlarge
図1. 上の画像は788 nmの単一波長の励起により取得しましたが、2つの蛍光タグの最適励起波長は750 nmと850 nmです。
波長可変フェムト秒レーザを使用した
高速切り替え
業界をリードする4000 nm/sの最高速度と、720~1060 nmの幅広いチューニングレンジのTiberius®Ti:サファイアフェムト秒レーザは、多光子顕微鏡用途における高速シーケンスイメージングに適しています。
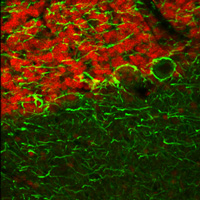
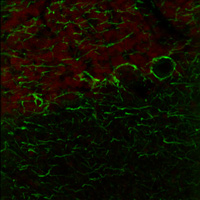
右の画像ならびに動画はラット成体の脳(厚さ25 µmの矢状断切片)です。赤のチャンネルは、835 nmの最適波長で励起されたニワトリ抗ニューロフィラメント抗体の蛍光像で、緑は750 nmの最適波長で励起されたマウス抗GFAP抗体の蛍光像です。
図1は788 nmの単一波長で励起した蛍光像で、2つの蛍光タグの同時励起では最適な像が得られないことを示しています。図2は、7 fpsで取得した2色励起イメージングシーケンスの合成画像を示します。励起波長は750~ 835 nmで素早くチューニングされました。図3の動画では、図2で合成画像の生成に使用された高速切り替えを1/16倍の速度でご覧いただけます。同じ強度で788 nmの単一波長の励起と比較した場合、両方の蛍光色素に適した励起が可能な高速切り替えの方が、より高い画像コントラストを得られています。
免疫蛍光の試料ご提供:Lynne Holtzclaw of the NICHD Microscopy and Imaging Core Facility, a part of the National Institutes of Health (NIH) in Bethesda, MD.
Click to Enlarge
図2.ガウシアンビーム
Click to Enlarge
図1. ベッセルビーム
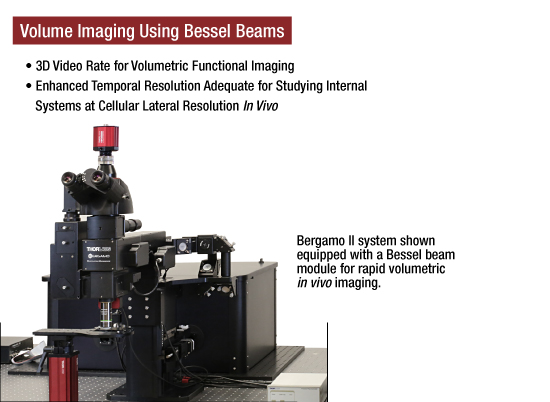
ベッセルビームを使用したボリュームイメージング手法
こちらは、ベッセルビームを使用した当社の新しい超高速イメージング手法です。神経回路や相互作用のin vivoボリューム機能イメージングをビデオレートで実現します。この独特なビームは、非回折で自己修復性をもつため、強く集光され、組織を透過しても再成形されます。この技術は、当社のBergamo II多光子顕微鏡と2光子メゾスコープに採用されます。
右はベッセルビームとガウシアンビームの画像です。画像でご覧いただけるように、ガウシアンビームの焦点は1点で、中心から広がるにつれ集光は弱まります。一方ベッセルビームは焦点を保つ円環ビームとなります。
こちらの技術に関するプレスリリースはこちらからご覧いただけます。
Click to Enlarge
回転式Bergamo IIシリーズシステムには多関節式ペリスコープが付いています。このペリスコープの設計により、走査システム全体を試料に対して傾けられるなど、柔軟性が強化されています。

Click to Enlarge
正立顕微鏡型Bergamo IIシリーズシステムには、光学性能を損なうことなく顕微鏡のXYZ各軸の全移動範囲が使用可能となるペリスコープが付いています。
ペリスコープ
多光子顕微鏡に用いられるレーザのほとんどは自由空間ビームとして伝播します。ビームのアライメントならびに最適化状態を維持するためにはビーム光路が固定されていなければなりません。Bergamo IIの焦点を中心に対物レンズを最大4軸方向(X、Y、 Z、θ)に移動できる機能においてビーム光路も同じ軸に沿って移動しなければなりません。Bergamo IIシリーズシステムは多関節式のペリスコープを用いることによりこの課題を克服しています。

Click to Enlarge
吸収フィルタとダイクロイックキューブは、PMT検出モジュールの前にある磁石で密閉されたドアの向こう側で保持されています。
スーパー広帯域補正走査光学系
Bergamo IIシリーズ顕微鏡には当社独自の走査用光学素子を使用しています。この光学素子は450~1100 nm、680~1300 nm、または800~1800 nmの励起波長用に最適化・補正されているため、それぞれ光刺激、2光子イメージング、そして3光子イメージングに適しています。可視域から近赤外域までのこの広い波長範囲は、最新の広帯域波長可変Ti:サファイアレーザ、OPOシステム、そしてChameleon Discoveryのような2波長出力レーザに対応できるよう選ばれています。
当社の光学素子は多光子顕微鏡では主力となる低倍率、高NAの対物レンズの光学設計を最大限に活用しています。対物レンズの後方開口部に入射ビームサイズを最大Ø20 mmまで合わせることができます。広い走査エリアにより、素早く関心領域(ROI)が特定でき、1度にイメージングできる細胞の数が多くなります。
集光角度の広い光学系
検出システムの最も重要な目標は数の限られた光子からより多くの信号を得ることです。PMTを対物レンズのすぐ後ろに配置すること(「ノンデスキャン」の幾何学的配置)により試料によって散乱する光は、対物レンズの視野外で起こってもPMTに当たって信号に加えられます。これは多光子顕微鏡独自の機構です。対物レンズの設計視野以外からも集光することにより、組織深部をイメージングする際、全体の検出効率を大幅に向上させています。
反射方向においては集光角度aが8°、10°または14°の光学系(入射瞳Ø20 mmに対し)、透過方向においては集光角度aが13°の光学系をご用意しております。集光モジュールに機械式シャッタを取り付けて光刺激の実験に用いることもできます。
アクセスが簡単な吸収フィルタならびにダイクロイックホルダ
Bergamo IIシリーズ顕微鏡システムは、Ø25 mm蛍光フィルタならびに25 mm x 36 mmダイクロイックミラーが入っている業界標準の蛍光フィルターセットに対応します。当社の検出モジュールは他社設計とは異なり磁石固定式のホルダとなっているため、素早く簡単にフィルタを交換して異なる測定が行えます。
当社では広域用Ø32 mm蛍光フィルタならびに32 mm x 44 mmダイクロイックミラー用の検出モジュールもご用意しており、より大きい集光角度で多くの信号検出が可能です。
反射ならびに透過ディテクタ
当社の多光子システムには、高感度GaAsP PMTを採用しています。これらのディテクタは量子効率が高く、蛍光強度が低い、または感光性が高い試料のイメージングに役立ちます。PMTは2種類ご用意しております。弱い信号に対して感度を改善した熱電冷却型PMT、そしてコンパクトで開口部が大きい非冷却型PMTです。Multialkali PMTもご用意しております。
すべてのBergamo® IIシリーズ顕微鏡は、反射(後)方向に2つまたは4つの検出器、さらに・または透過(前)方向に2つの検出器を取り付けることが可能です。透過(前)方向に検出チャンネルを構成することで、反射方向に設定したPMTと同一の蛍光タグを検出することができ、薄くて蛍光強度が低い試料に対して顕微鏡の感度を向上させることが可能です。
ソフトウェアにより1度で最大4チャンネルが制御できます。
タッチスクリーン付き多軸コントローラ
こちらのコントローラは回転式のBergamo IIシリーズ顕微鏡本体用に設計されています。ノブで最大5軸を電動制御します。回転式システムでは、対物レンズ焦点の微調整と筐体の昇降によるz軸移動をロッカースイッチにより切り替えます。ご希望の筐体位置を維持したい場合には、各軸の移動操作を個別にロックすることができます。
タッチスクリーンを用いて2つの位置を保存、読み出しできます。PCのThorImage®LS上では、最大8つの位置を保存可能です。タッチスクリーンは、すべてのモータの位置の読み出しも行います。
対物レンズ
Bergamo IIシリーズ顕微鏡は、M34 x 1.0、M32 x 0.75、M25 x 0.75ならびにRMSネジ付きの無限遠補正対物レンズに対応します。これにより多光子顕微鏡に使用される多くの低倍率、高NA対物レンズを装置に取り付けることが可能になります。当社の走査用光学素子は視野数が20と大きく、これらの多光子イメージング用対物レンズの光学設計を利用することで、同じ対物レンズを使用した他社製顕微鏡と比べても集光性能が高くなっています。
高剛性スタンドサンプルホルダ
当社の高剛性スタンドサンプルホルダは、回転や固定が可能な薄型プラットフォームで、スライド、記録チャンバ、Z軸ピエゾステージやカスタム仕様の実験器具を取り付けます。どの部品もパッシブ振動減衰のためØ38 mm(Ø1.5インチ)ステンレススチール製ポストで支えられ、さらに赤いポストホルダによってワークステーションなどに固定されます。
試料設置プラットフォームの高さはロック用カラーで保持されており、回転させることにより簡単に光路上に置いたり、離したりすることができます。また、位置が決まった後は、クイックリリース機構によりポストが固定されます。

Click to Enlarge
sCMOSカメラZeluxとQuantulux
サイエンティフィックカメラ
低ノイズの sCMOSならびにCMOSカメラ は、当社の多光子顕微鏡システムに完全対応するよう設計されました。ワイドフィールドならびに蛍光顕微鏡における観察・撮像で、反射光ならびに蛍光発光でin vitroならびにin vivo試料を可視化することができます。落射蛍光照明モジュールとともに使用することにより基準マーカを特定するのに役に立ちます。また、レーザ照射が不要なイメージング手法にもお使いいただけます。
当社のサイエンティフィックカメラは、自社開発のThorCamソフトウェアパッケージで作動し、sCMOSカメラは2.1 メガピクセル、CMOSカメラは1.3、2.3、5、8.9、12.3メガピクセルセンサでご用意しております。 一般的にカメラの解像度が低いと最大フレームレートが高くなります。これらのカメラには、外部トリガ信号により画像取得が可能な補助ポートも付いています。
Bergamo® IIシリーズ顕微鏡は、業界標準のCマウントならびにCSマウントネジ付きのカメラにも対応しています。

Click to Enlarge
対物レンズの下にある透過モジュール、電動式コンデンサ、そして高剛性スタンドサンプルホルダ
お客様ご自身でDodtコントラストならびにDICイメージングモジュールを取り付け可能
Bergamo® IIシリーズのモジュール式構成により、in vitroならびにin vivoでの実験の切り替えが非常に簡単になります。お客様ご自身で取り付けが可能なDodtコントラスト、レーザ走査によるDodtコントラスト、DICモジュールは本体への取り付け、取り外しが5分未満で行えます。モジュールは回転式ならびに正立顕微鏡型本体用の両方をご用意しております。
各製品には当社の3軸コントローラが付属し、12.7 mmの範囲で電動式コンデンサを移動させることで照明条件を最適化します。この多機能デザインによりNikon製コンデンサや高NA油浸コンデンサにも対応します。
これらのモジュールを補完するため、当社では透過照明モジュールと対物レンズ間にスライドを適切に配置できる薄型高剛性スタンドサンプルホルダを製造しています。
当社では、用途に応じて様々なご要望にお応えできるように、
お客様のニーズに合わせたご提案を心掛けています。
ご意見・ご要望、またご質問などございましたら当社までお気軽にご連絡ください。
Thorlabs' Bergamo® II Series Multiphoton Microscopy Platform is a powerful tool adapted to meet experimental needs across a wide range of research fields. Click on the images below to explore how a Bergamo can and has been utilized for each application.
構成例
下表ではBergamo IIの回転式、XYZ軸ならびにZ軸システム構成例の詳細がご覧いただけます。当社の多光子顕微鏡プラットフォームはモジュール設計により、それぞれの実験ニーズに合った構成に変更したり、設置後の顕微鏡機能の調整が可能です。各モジュールについての詳細は「モジュール」タブをご覧ください。
| 構成 | システムの概要 | ||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 回転式筐体 | |||||||||||||||||||||||||||||||||||||||||||||
| 構成B243: 同時多点光刺激 | 主な特長 | 推奨する用途 | |||||||||||||||||||||||||||||||||||||||||||
|
| ||||||||||||||||||||||||||||||||||||||||||||
同時多点光刺激構成の仕様詳細
当社の空間変調モジュール(SLM)は、レーザービームの位相を操作することにより何百もの集光点をユーザが望む位置に生じさせます。SLMの位相マスクのパターンは素早く切り替え可能であり、個々の焦点を同時に刺激可能です。刺激用ビームを成形し、光刺激の効果を向上させることができます。これは、単一視野内の異なる深度で神経集団を活性化させるのに重要な機能です。 こちらの構成ではイメージングと光刺激用に別々の2つのレーザを使用しています。Bergamo顕微鏡システムは高度な構成柔軟性を持ち、デュアル出力光源向けに光路を最適化することができます。  Click to Enlarge SLMは副光路に設置されており、多光子イメージングと同時進行で光刺激が可能です。  Click to Enlarge 多光子イメージングにはTiberius高速切り替えチューナブルレーザ、光刺激にはMenlo SystemのBlueCutファイバーレーザを使用しています。 | |||||||||||||||||||||||||||||||||||||||||||||
| 構成B242: 2光子または3光子イメージング | 主な特長 | 推奨する用途 | |||||||||||||||||||||||||||||||||||||||||||
|
| ||||||||||||||||||||||||||||||||||||||||||||
2光子または3光子イメージング構成の仕様詳細
こちらのデュアル走査の構成は、ガルバノ-レゾナントとガルバノ-ガルバノの両方のスキャナと、近赤外走査素子を第2次ならびに第3次高調波発生(SHGならびにTHG)イメージングに使用します。  Click to Enlarge 主光路ならびに副光路の光学素子はそれぞれ2光子と3光子イメージング用に最適化されています。ビーム路は、同時のマルチチャンネルイメージング用に最適化されています。  Click to Enlarge 当社の回転式顕微鏡ベースで対象物を最大+90°の角度でご覧ください。顕微鏡ボディの回転は対物レンズの焦点が軸となっているため、焦点の再調整の必要なく自由な回転が可能です。 | |||||||||||||||||||||||||||||||||||||||||||||
| 構成B251: ランダムアクセス走査 | 主な特長 | 推奨する用途 | |||||||||||||||||||||||||||||||||||||||||||
|
| ||||||||||||||||||||||||||||||||||||||||||||
ランダムアクセス走査構成の仕様詳細こちらの高速ランダムアクセス走査構成は、レゾナント-ガルバノ-ガルバノスキャナを使用して、単一視野内で複数の高分解能画像を取得します。スキャナは、ユーザ定義の領域を複数選択可能なうえ、レゾナント-ガルバノスキャナの速度で動作します。このイメージング機能は、脳の複数の部位での神経応答の相関解析に適しています。
 Click to Enlarge 聴覚神経の観察などにはこちらのようにサウンドボックス内に顕微鏡を設置することができます。 | |||||||||||||||||||||||||||||||||||||||||||||
| 構成B241: in vivo 2光子イメージング | 主な特長 | 推奨する用途 | |||||||||||||||||||||||||||||||||||||||||||
|
| ||||||||||||||||||||||||||||||||||||||||||||
In Vivo2光子イメージング構成の仕様詳細こちらの回転式のBergamo顕微鏡は、コンパクトな構成でエンクロージャ内に納めることができるため、音や光に敏感なin vivo観察を行うことができます。
 Click to Enlarge サウンドボックス内の2光子イメージング用回転式顕微鏡 | |||||||||||||||||||||||||||||||||||||||||||||
| 正立型顕微鏡(XYZ) | |||||||||||||||||||||||||||||||||||||||||||||
| 構成B252: デュアル光路ランダムアクセス走査 | 主な特長 | 推奨する用途 | |||||||||||||||||||||||||||||||||||||||||||
|
| ||||||||||||||||||||||||||||||||||||||||||||
デュアル光路ランダムアクセス走査構成の仕様詳細
ランダムアクセス走査用に装備されているこちらの構成は、主光路にレゾナント-ガルバノ-ガルバノスキャナ、副光路にガルバノ-ガルバノスキャナの両方を組み込んでいます。システムは多光子イメージング用にデュアル出力レーザを使用しています。  Click for Details 上の大型のGibraltarステージにより、試料と補助機器の両方が対物レンズの焦点面にアライメントできます。こちらでは、パッチクランプ記録用に細胞が操作できるよう電動マイクロマニピュレータをブレッドボードに取り付けています。  Click to Enlarge カスタムエンクロージャをビーム調整モジュールやほかの部品に使用すれば、遮光領域ができ、ほかのビーム路に影響することなく部品の調整が可能となります。  Click to Enlarge 主光路のレゾナント-ガルバノ-ガルバノスキャナは、単一視野内で複数の関心領域が素早く走査可能となり、副光路のガルバノ-ガルバノスキャナは、同時光刺激にご使用いただけます。 | |||||||||||||||||||||||||||||||||||||||||||||
| 構成B262:共焦点イメージング用デュアル走査 | 主な特長 | 推奨する用途 | |||||||||||||||||||||||||||||||||||||||||||
|
| ||||||||||||||||||||||||||||||||||||||||||||
デュアル走査共焦点構成の仕様詳細
こちらの複数走査路とモダリティの構成は、光刺激とともに多光子、共焦点ならびに落射蛍光イメージングのあらゆる組み合わせを使用した様々な実験条件で観察が可能です。光刺激と多光子イメージングの組み合わせは、主光路でガルバノ-レゾナントスキャナによる高速イメージング、副光路でガルバノ-ガルバノスキャナによる特定の領域での光刺激を行う、2つの走査路で実施可能です。4チャンネル共焦点レーザは、ファイバのバルクヘッドから副光路に接続し、4チャンネル型PMT検出モジュールは対物レンズの後方焦点面にあるポートを使用して接続します。落射蛍光観察は最大6個のフィルタ用落射照明モジュールとsCMOS Quantaluxカメラの取り付けで実施可能です。 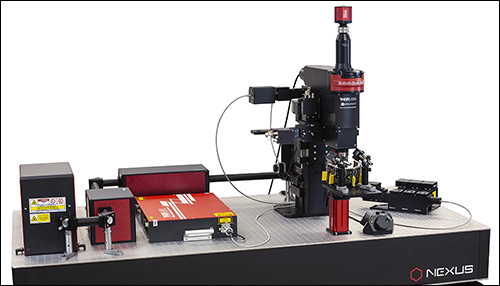 Click to Enlarge 当社では様々な試料向けの取付けオプションをご用意しております。剛性スタンドのブレッドボードインサートが写真のようにVRフライシアターと補助カメラを対物レンズの下に取り付けています。 | |||||||||||||||||||||||||||||||||||||||||||||
| 構成B231: 単純なイメージング(XYZ) | 主な特長 | 推奨する用途 | |||||||||||||||||||||||||||||||||||||||||||
|
| ||||||||||||||||||||||||||||||||||||||||||||
単純なイメージング(XYZ)の仕様詳細
こちらの構成は in vitro ならびに固定式ステージのin vivo顕微鏡観察のどちらにも適しています。脱着式の透過光モジュール付きのシステムは、さまざまな実験手法、イメージング手法、そして対象物に対応する汎用性があります。  Click to Enlarge 顕微鏡下の広い空間により、補助機器を使用した大型のセットアップを対物レンズ下に設置しやすくなります。こちらでは、ショウジョウバエ用のVRシアターステージと2つの補助カメラを取り付けています。セットアップは、ThorSync(ThorImageLS)ソフトウェアのアドオン)を使用した画像取得機能と同期可能です。  Click to Enlarge ほかのイメージング手法の信号検出向けに自由空間光用フォトディテクタをあらゆるPMTに交換することができます。 こちらは、PMTの代わりに反射光を検知するDET10A2を取り付けた顕微鏡です。 | |||||||||||||||||||||||||||||||||||||||||||||
| 正立型顕微鏡(Z軸) | |||||||||||||||||||||||||||||||||||||||||||||
| 構成B211: 動画ならびに高速イメージング | 主な特長 | 推奨する用途 | |||||||||||||||||||||||||||||||||||||||||||
|
| ||||||||||||||||||||||||||||||||||||||||||||
動画イメージング構成の仕様詳細
こちらの構成はビデオレートの2光子シーケンスイメージングで高速かつ動的な生物/.化学プロセスが観察可能です。  Click to Enlarge 高剛性スタンドは、こちらのショウジョウバエ の観察で使用されているマルチカメラのVRシアターを含め、様々な取り付けオプションに安定性をもたらします。  Click to Enlarge ガルバノ-レゾナントスキャナは8 kHzおよび12 kHzの共振周波数でご用意しております。 | |||||||||||||||||||||||||||||||||||||||||||||
| 構成B201: 単純なイメージング(Z) | 主な特長 | 推奨する用途 | |||||||||||||||||||||||||||||||||||||||||||
|
| ||||||||||||||||||||||||||||||||||||||||||||
単純なZ軸イメージング構成の仕様詳細こちらは試料面での多光子イメージングを要する単純な顕微鏡システムの構成で、不要な機能は付いていません。なおこちらの構成にはパワー減衰器が必要です(写真に含まれておりません)。
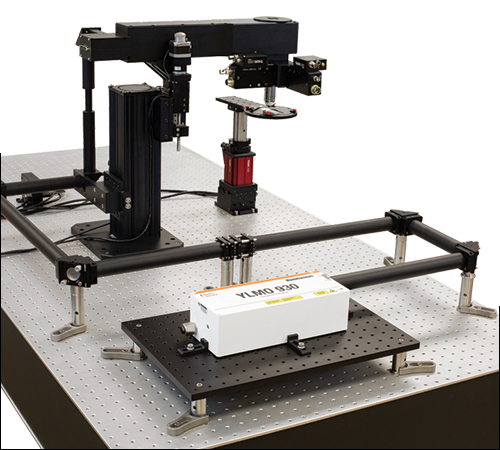 Click to Enlarge こちらではMenlo SystemのYLMO 930 nmレーザを主光源としています。イメージング素子は短波長あるいは長波長の多光子イメージング向けに調整可能で、ガルバノ-ガルバノスキャナはより大きいあるいは小さいビーム径に対応できるよう交換できます。 | |||||||||||||||||||||||||||||||||||||||||||||
当社では、用途に応じて様々なご要望にお応えできるように、お客様のニーズに合わせたご提案を心掛けています。
ご意見・ご要望、またご質問などございましたら当社までお気軽にご連絡ください。

Click to Enlarge
回転式Bergamo IIシステム用の透過光路モジュール
既存の多光子イメージングシステム向けの追加オプション
- 既存の顕微鏡基盤ならびに部品の機能アップグレード
- 部品の追加による機能向上
当社のモジュール設計の顕微鏡は、実験ニーズに応じて常に進化させることができます。旧モデルの顕微鏡や多光子顕微鏡製品も、必要に応じ、基盤のアップグレードや既存部品に機能を追加できる柔軟性が備わっています。当社の多光子システムのラインナップの追加オプションについては下表をご覧いただき、アップグレードもしくはアドオンの詳細については当社までご連絡ください。
なお、アップグレードやアドオンの設置には担当者が訪問させていただく場合があることをご了承ください。
| Bergamo IIに対応するアップグレードとアドオン |
|---|
| Bergamo I (B-Scope)に対応するアップグレードとアドオン |
|---|
| Acerra (A-Scope)に対応するアップグレードとアドオン |
|---|
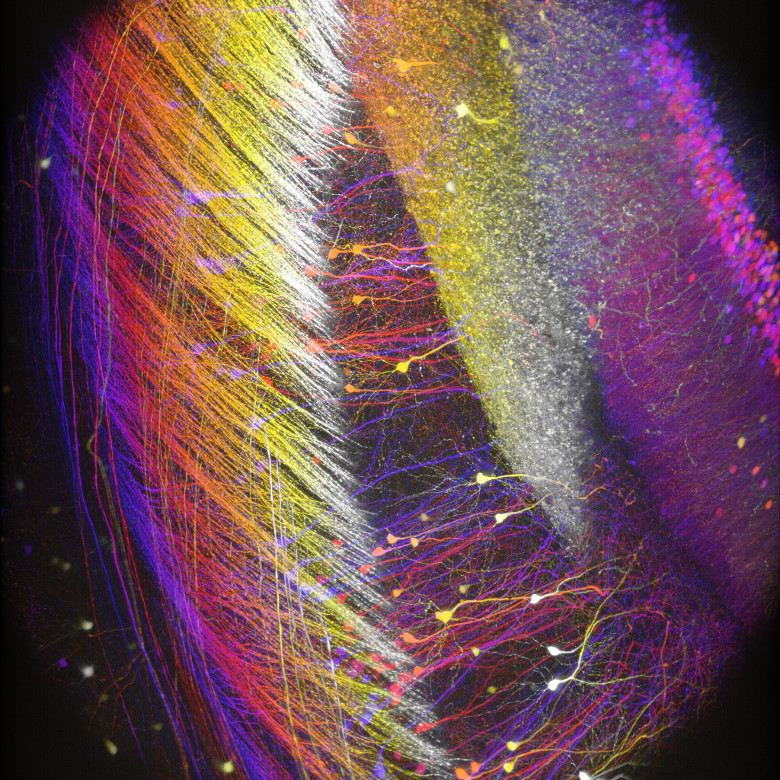
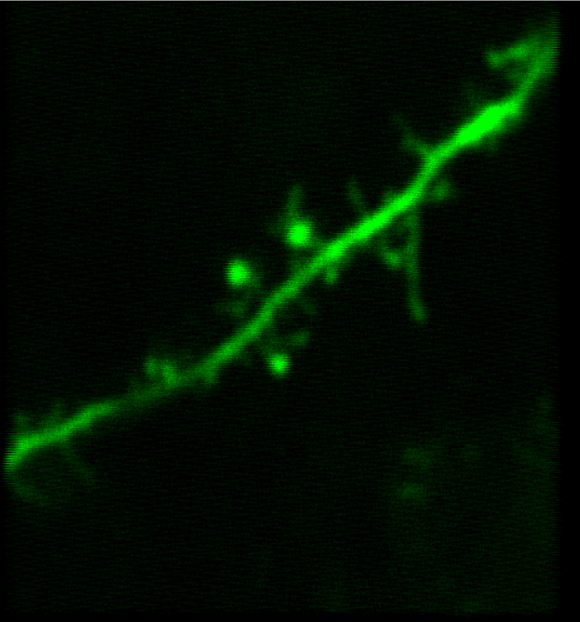
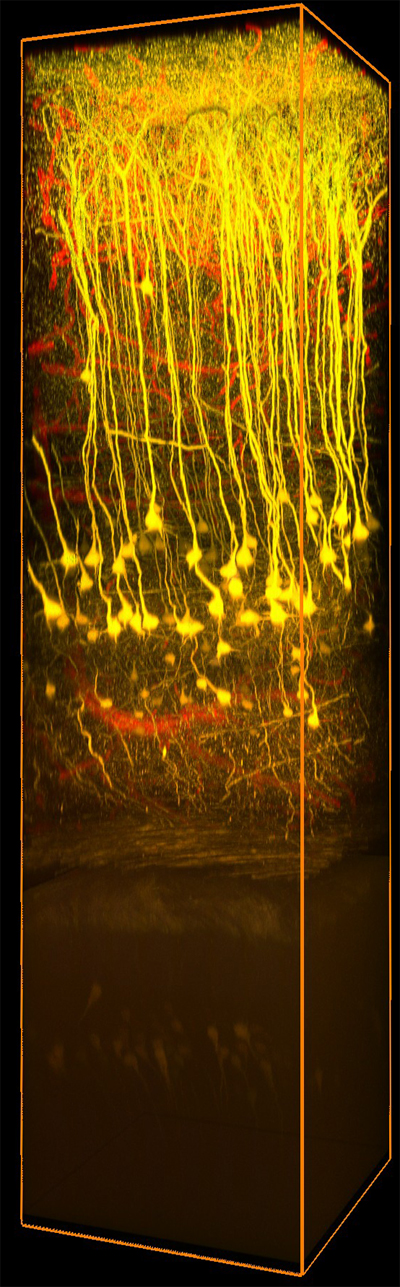
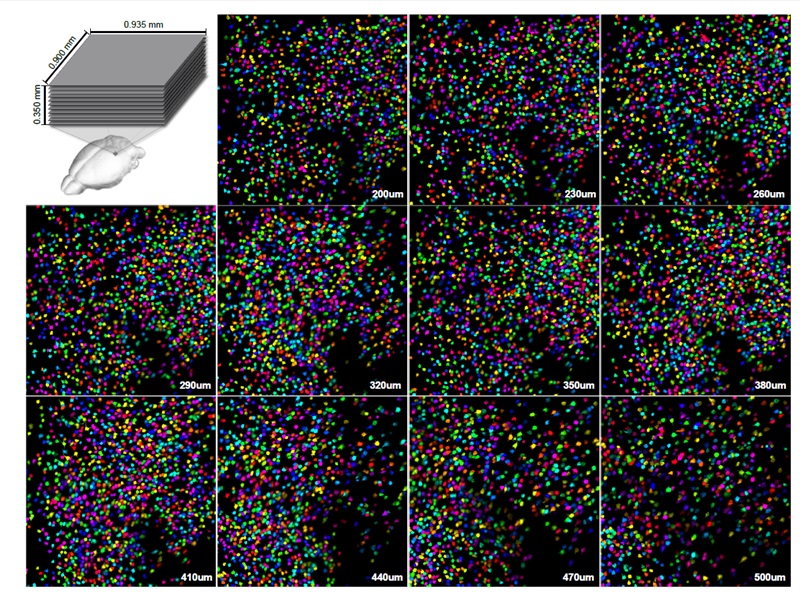
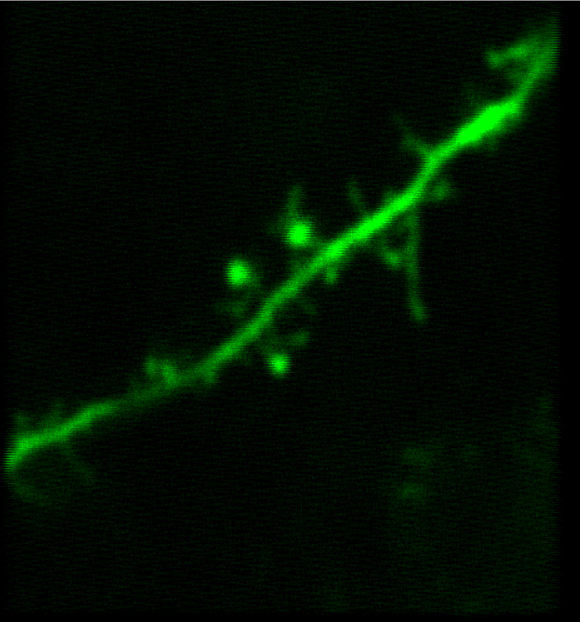
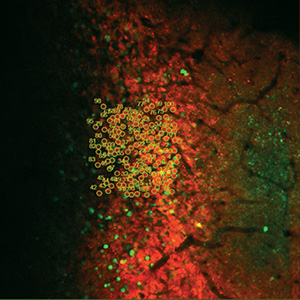
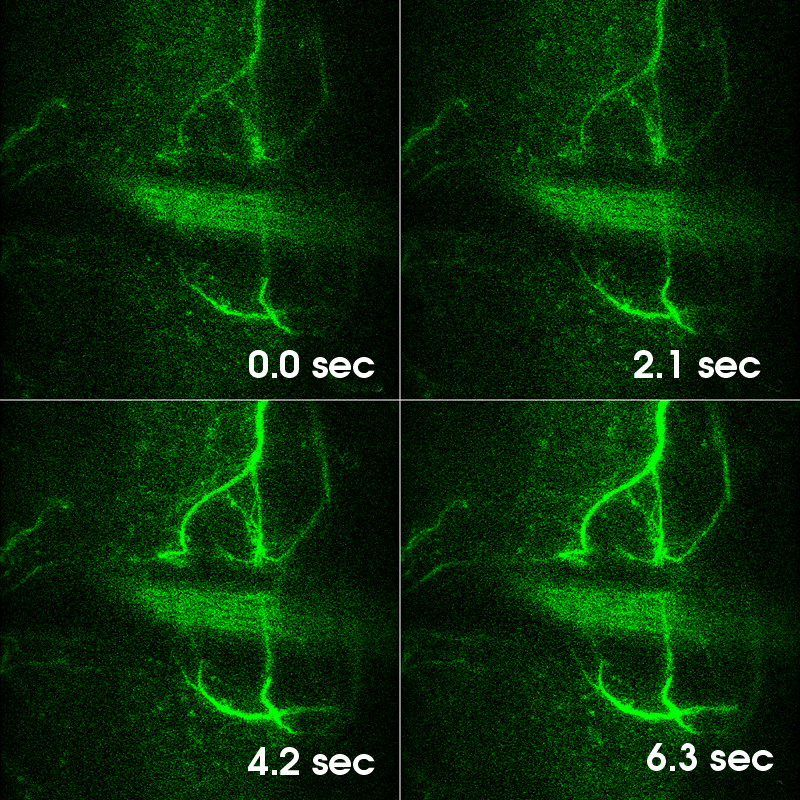
Images Taken Using Bergamo® Systems
Selected Publications Using Thorlabs' Imaging Systems
2023
Tsuji, M., Nishizuka, Y. & Emoto, K. (2023). Threat gates visual aversion via theta activity in Tachykinergic neurons. Nature Communications, 14(1), 3987.
Villanueva, C. B., Stephensen, H. J. T., Mokso, R., Benraiss, A., Sporring, J., Goldman, S.A. (2023). Astrocytic engagement of the corticostriatal synaptic cleft is disrupted in a mouse model of Huntington's disease. Proceedings of the National Academy of Sciences, 120(24), e2210719120.
Holstein-Rønsbo, S., Gan, Y., Giannetto, M.J. et al. (2023). Glymphatic influx and clearance are accelerated by neurovascular coupling. Nature Neuroscience, 26, 1042–1053.
Serradj, N., Marino, F., Moreno-López, Y. et al. (2023). Task-specific modulation of corticospinal neuron activity during motor learning in mice. Nature Communications, 14(1), 2708.
Inayat, S., McAllister, B. B., Whishaw, I. Q., & Mohajerani, M. H. (2023). Hippocampal conjunctive and complementary CA1 populations relate sensory events to movement. Iscience, 26(4), 106481.
Untiet, V., Beinlich, F. R., Kusk, P., Kang, N., Ladrón-de-Guevara, A., Song, W., ... & Nedergaard, M. (2023). Astrocytic chloride is brain state dependent and modulates inhibitory neurotransmission in mice. Nature Communications, 14(1), 1871.
Anthoney, N., Tainton-Heap, L., Luong, H., Notaras, E., Zhao, Q., Perry, T., ... & van Swinderen, B. (2023). Experimentally induced active and quiet sleep engage non-overlapping transcriptomes in Drosophila. bioRxiv, 535331.
Boster, K. A., Cai, S., Ladrón-de-Guevara, A., Sun, J., Zheng, X., Du, T., ... & Kelley, D. H. (2023). Artificial intelligence velocimetry reveals in vivo flow rates, pressure gradients, and shear stresses in murine perivascular flows. Proceedings of the National Academy of Sciences, 120(14), e2217744120.
Scarpetta, V., Bodaleo, F., Salio, C., Agarwal, A., Sassoè-Pognetto, M., & Patrizi, A. (2023). Morphological and mitochondrial changes in murine choroid plexus epithelial cells during healthy aging. Fluids and Barriers of the CNS, 20(1), 1-16.
McDermott, K. D., Frechou, M. A., Jordan, J. T., Martin, S. S., & Gonçalves, J. T. (2023). Delayed formation of neural representations of space in aged mice. bioRxiv, 531021.
Matthews, M. D., Cook, E., Naguib, ,N., Wiesner, U. B., & Lewis, K. J. (2023). Intravital imaging of osteocyte integrin dynamics with locally injectable fluorescent nanoparticles. Bone, 174, 116830.
Tort-Colet, N., Resta, F., Montagni, E., Pavone, F., Allegra Mascaro, A. L., & Destexhe, A. (2023). Assessing brain state and anesthesia level with two-photon calcium signals. Scientific Reports, 13(1), 3183.
Esteves, I. M., Chang, H., Neumann, A. R., & McNaughton, B. L. (2023). Consolidation of cellular memory representations in superficial neocortex. Iscience, 26(2), 105970.
Quezada, A., Ward, C., Bader, E. R., Zolotavin, P., Altun, E., Hong, S., ... & Hébert, J. M. (2023). An in vivo platform for rebuilding functional neocortical tissue. Bioengineering, 10(2), 263.
Yang, J. Y., O’Connell, T. F., Hsu, W. M. M., Bauer, M. S., Dylla, K. V., Sharpee, T. O., & Hong, E. J. (2023). Restructuring of olfactory representations in the fly brain around odor relationships in natural sources. bioRxiv, 528627.
Carlton, A. J., Jeng, J. Y., Grandi, F. C., De Faveri, F., Ceriani, F., De Tomasi, L., ... & Marcotti, W. (2023). A critical period of prehearing spontaneous Ca2+ spiking is required for hair-bundle maintenance in inner hair cells. The EMBO Journal, 42(4), e112118.
Hamling, K. R., Zhu, Y., Auer, F., & Schoppik, D. (2023). Tilt In Place Microscopy (TIPM): a simple, low-cost solution to image neural responses to body rotations. Journal of Neuroscience, 43(6), 936-948.
Vestergaard, M., Carta, M., Güney, G., & Poulet, J. F. A. (2023). The cellular coding of temperature in the mammalian cortex. Nature, 614(7949), 725-731.
Contreras-López, R., Alatriste-León, H., Díaz-Hernández, E., Ramírez-Jarquín, J. O., & Tecuapetla, F. (2023). The deep cerebellar nuclei to striatum disynaptic connection contributes to skilled forelimb movement. Cell Reports, 42(1), 112000.
Mabuchi, Y., Cui, X., Xie, L., Kim, H., Jiang, T., & Yapici, N. (2023). GABA-mediated inhibition in visual feedback neurons fine-tunes Drosophila male courtship. bioRxiv, 525544.
Das, A., Margevicius, D., Borovicka, J., Icardi, J., Patel, D., Paquet, M. E., & Dana, H. (2023). Enhanced detection sensitivity of neuronal activity patterns using CaMPARI1 vs. CaMPARI2. Frontiers in Neuroscience, 16, 2291.
Lee, S., Lee, K., Choi, M., & Park, J. (2023). Implantable acousto-optic window for monitoring ultrasound-mediated neuromodulation in vivo. Neurophotonics, 9(3), 032203.
Zhuo, G. Y., Chen, M. C., Lin, T. Y., Lin, S. T., Chen, D. T. L., & Lee, C. W. S. (2023). Opioid-Modulated Receptor Localization and Erk1/2 Phosphorylation in Cells Coexpressing μ-Opioid and Nociceptin Receptors. International Journal of Molecular Sciences, 24(2), 1048.
Diaz-Cuadros, M., Miettinen, T. P., Skinner, O. S., Sheedy, D., Díaz-García, C. M., Gapon, S., ... & Pourquié, O. (2023). Metabolic regulation of species-specific developmental rates. Nature, 613(7944), 550-557.
Evrard, M., Mansuryan, T., Couderc, V., Désévédavy, F., Strutynski, C., Dussauze, M., ... & Smektala, F. (2023). Highly Nonlinear Multimode Tellurite Fibers: From Glass Synthesis to Practical Applications in Multiphoton Imaging. Advanced Photonics Research, 4(1), 2200213.
2022
Graham, R. T., Parrish, R. R., Alberio, L., Johnson, E. L., Owens, L., & Trevelyan, A. J. (2022). Optogenetic stimulation reveals a latent tipping point in cortical networks during ictogenesis. Brain, awac487.
Shiozaki, H. M., Wang, K., Lillvis, J. L., Xu, M., Dickson, B. J., & Stern, D. L. (2022). Neural coding of distinct motor patterns during Drosophila courtship song. bioRxiv, 520499.
Fisher, Y. E., Marquis, M., D’Alessandro, I., & Wilson, R. I. (2022). Dopamine promotes head direction plasticity during orienting movements. Nature, 612(7939), 316-322.
Wang, X., Delle, C., Asiminas, A., Akther, S., Vittani, M., Brxxxgger, P., ... & Hirase, H. (2022). Liver-secreted fluorescent blood plasma markers enable chronic imaging of the microcirculation. Cell Reports Methods, 2(10), 100302.
Lu, Y., Wei, X., Li, W., Wu, X., Chen, C., Li, G., ... & Gan, W. B. (2022). Large-volume and deep brain imaging in rabbits and monkeys using COMPACT two-photon microscopy. Scientific Reports, 12(1), 17736.
Huang, J. S., Kunkhyen, T., Rangel, A. N., Brechbill, T. R., Gregory, J. D., Winson-Bushby, E. D., ... & Cheetham, C. E. (2022). Immature olfactory sensory neurons provide behaviourally relevant sensory input to the olfactory bulb. Nature Communications, 13(1), 6194.
Govindaraju, I., Muraleedharan, M., Maidin, S., Chakraborty, I., Zhuo, G. Y., Mahato, K. K., & Mazumder, N. (2022). Investigation of the effect of gamma irradiation on the morphological structures of starch using advanced microscopic techniques. In Frontiers in Optics, JTu5A-74.
Hwang, F. J., Roth, R. H., Wu, Y. W., Sun, Y., Kwon, D. K., Liu, Y., & Ding, J. B. (2022). Motor learning selectively strengthens cortical and striatal synapses of motor engram neurons. Neuron, 110(17), 2790-2801.
Yue, Y., Ash, R. T., Boyle, N., Kinter, A., Li, Y., Zeng, C., & Lu, H. (2022). MeCP2 deficiency impairs motor cortical circuit flexibility associated with motor learning. Molecular Brain, 15(1), 1-12.
Liu, Z., Lu, X., Villette, V., Gou, Y., Colbert, K. L., Lai, S., Guan, S., ... & St-Pierre, F. (2022). Sustained deep-tissue voltage recording using a fast indicator evolved for two-photon microscopy. Cell, 185(18), 3408-3425.
Aragon, M. J., Mok, A. T., Shea, J., Wang, M., Kim, H., Barkdull, N., ... & Yapici, N. (2022). Multiphoton imaging of neural structure and activity in Drosophila through the intact cuticle. Elife, 11, e69094.
Matheson, A. M., Lanz, A. J., Medina, A. M., Licata, A. M., Currier, T. A., Syed, M. H., & Nagel, K. I. (2022). A neural circuit for wind-guided olfactory navigation. Nature Communications, 13(1), 4613.
Chornyy, S., Borovicka, J. A., Patel, D., Shin, M. K., Vázquez-Rosa, E., Miller, E., ... & Dana, H. (2022). Longitudinal in vivo monitoring of axonal degeneration after brain injury. Cell Reports Methods, 3(5), 100481.
Sheng, W., Zhao, X., Huang, X., & Yang, Y. (2022). Real-time image processing toolbox for all-optical closed-loop control of neuronal activities. Frontiers in Cellular Neuroscience, 16, 917713.
Tadres, D., Shiozaki, H. M., Tastekin, I., Stern, D. L., & Louis, M. (2022). An essential experimental control for functional connectivity mapping with optogenetics. bioRxiv, 493610.
Koveal, D., Rosen, P. C., Meyer, D. J., Díaz-García, C. M., Wang, Y., Cai, L. H., ... & Yellen, G. (2022). A high-throughput multiparameter screen for accelerated development and optimization of soluble genetically encoded fluorescent biosensors. Nature Communications, 13(1), 2919.
Fukuda, M., Matsumura, T., Suda, T., & Hirase, H. (2022). Depth-targeted intracortical microstroke by two-photon photothrombosis in rodent brain. Neurophotonics, 9(2), 021910-021910.
Jackson, J. G., Krizman, E., Takano, H., Lee, M., Choi, G. H., Putt, M. E., & Robinson, M. B. (2022). Activation of Glutamate Transport Increases Arteriole Diameter in vivo: Implications for Neurovascular Coupling. Frontiers in Cellular Neuroscience, 16, 831061.
Du, T., Mestre, H., Kress, B. T., Liu, G., Sweeney, A. M., Samson, A. J., ... & Nedergaard, M. (2022). Cerebrospinal fluid is a significant fluid source for anoxic cerebral oedema. Brain, 145(2), 787-797.
Govindaraju, I., Zhuo, G. Y., Chakraborty, I., Melanthota, S. K., Mal, S. S., Sarmah, B., ... & Mazumder, N. (2022). Investigation of structural and physico-chemical properties of rice starch with varied amylose content: A combined microscopy, spectroscopy, and thermal study. Food Hydrocolloids, 122, 107093.
Choe, K., Hontani, Y., Wang, T., Hebert, E., Ouzounov, D. G., Lai, K., ... & Xu, C. (2022). Intravital three-photon microscopy allows visualization over the entire depth of mouse lymph nodes. Nature Immunology, 23(2), 330-340.
Spivak, Y. M., & Lemeshko, P. S. (2022). Multiphoton microscopy of mesoporous silicon. In Journal of Physics: Conference Series. 2227(1), 012013.
2021
Maset, A., Albanesi, M., di Soccio, A., Canova, M., Dal Maschio, M., & Lodovichi, C. (2021). Aberrant Patterns of Sensory-Evoked Activity in the Olfactory Bulb of LRRK2 Knockout Mice. Cells, 10(11), 3212.
Schmidt, E. R., Zhao, H. T., Park, J. M., Dipoppa, M., Monsalve-Mercado, M. M., Dahan, J. B., ... & Polleux, F. (2021). A human-specific modifier of cortical connectivity and circuit function. Nature, 599(7886), 640-644.
Hermans, L., Kaynak, M., Braun, J., Lobato Ríos, V., Chen, C. L., Günel, S., ... & Ramdya, P. (2021). Long-term imaging of the ventral nerve cord in behaving adult Drosophila. bioRxiv, 463778.
Carlsen, E. M. M., Falk, S., Skupio, U., Robin, L., Pagano Zottola, A. C., Marsicano, G., & Perrier, J. F. (2021). Spinal astroglial cannabinoid receptors control pathological tremor. Nature Neuroscience, 24(5), 658-666.
Moussa, N. O., Mansuryan, T., Hage, C. H., Fabert, M., Krupa, K., Tonello, A., ... & Couderc, V. (2021). Spatiotemporal beam self-cleaning for high-resolution nonlinear fluorescence imaging with multimode fiber. Scientific Reports, 11(1), 18240.
Eleftheriou, C. G., Corona, C., Khattak, S., Alam, N. M., Ivanova, E., Bianchimano, P., ... & Sagdullaev, B. T. (2021). Retinoschisin deficiency induces persistent aberrant waves of activity affecting neuroglial signaling in the retina. Journal of Neuroscience, 42(36), 6983-7000.
Han, J., Kim, S., Choi, P., Lee, S., Jo, Y., Kim, E., & Choi, M. (2021). Robust functional imaging of taste sensation with a Bessel beam. Biomedical Optics Express, 12(9), 5855-5864.
Favuzzi, E., Huang, S., Saldi, G. A., Binan, L., Ibrahim, L. A., Fernández-Otero, M., ... & Fishell, G. (2021). GABA-receptive microglia selectively sculpt developing inhibitory circuits. Cell, 184(15), 4048-4063.
Chornyy, S., Das, A., Borovicka, J. A., Patel, D., Chan, H. H., Hermann, J. K., ... & Dana, H. (2021). Cellular-resolution monitoring of ischemic stroke pathologies in the rat cortex. Biomedical Optics Express, 12(8), 4901-4919.
Malina, K. C. K., Tsivourakis, E., Kushinsky, D., Apelblat, D., Shtiglitz, S., Zohar, E., ... & Spiegel, I. (2021). NDNF interneurons in layer 1 gain-modulate whole cortical columns according to an animal’s behavioral state. Neuron, 109(13), 2150-2164.
Bruzzone, M., Chiarello, E., Albanesi, M., Miletto Petrazzini, M. E., Megighian, A., Lodovichi, C., & Dal Maschio, M. (2021). Whole brain functional recordings at cellular resolution in zebrafish larvae with 3D scanning multiphoton microscopy. Scientific Reports, 11(1), 11048.
Mridha, Z., de Gee, J. W., Shi, Y., Alkashgari, R., Williams, J., Suminski, A., ... & McGinley, M. J. (2021). Graded recruitment of pupil-linked neuromodulation by parametric stimulation of the vagus nerve. Nature Communications, 12(1), 1539.
Ivanova, E., Corona, C., Eleftheriou, C. G., Bianchimano, P., & Sagdullaev, B. T. (2021). Retina-specific targeting of pericytes reveals structural diversity and enables control of capillary blood flow. Journal of Comparative Neurology, 529(6), 1121-1134.
Hung, C. W., Mazumder, N., Lin, D. J., Chen, W. L., Lin, S. T., Chan, M. C., & Zhuo, G. Y. (2021). Label-free characterization of collagen crosslinking in bone-engineered materials using nonlinear optical microscopy. Microscopy and Microanalysis, 27(3), 587-597.
Hontani, Y., Xia, F., & Xu, C. (2021). Multicolor three-photon fluorescence imaging with single-wavelength excitation deep in mouse brain. Science Advances, 7(12), eabf3531.
Resnik, J., & Polley, D. B. (2021). Cochlear neural degeneration disrupts hearing in background noise by increasing auditory cortex internal noise. Neuron, 109(6), 984-996.
Tainton-Heap, L. A., Kirszenblat, L. C., Notaras, E. T., Grabowska, M. J., Jeans, R., Feng, K., ... & van Swinderen, B. (2021). A paradoxical kind of sleep in Drosophila melanogaster. Current Biology, 31(3), 578-590.
Hortholary, T., Carrion, C., Chouzenoux, E., Pesquet, J. C., & Lefort, C. (2021). Multiplex-multiphoton microscopy and computational strategy for biomedical imaging. Microscopy Research and Technique, 84(7), 1553-1562.
Clayton, K. K., Williamson, R. S., Hancock, K. E., Tasaka, G. I., Mizrahi, A., Hackett, T. A., & Polley, D. B. (2021). Auditory corticothalamic neurons are recruited by motor preparatory inputs. Current Biology, 31(2), 310-321.
Esteves, I. M., Chang, H., Neumann, A. R., Sun, J., Mohajerani, M. H., & McNaughton, B. L. (2021). Spatial information encoding across multiple neocortical regions depends on an intact hippocampus. Journal of Neuroscience, 41(2), 307-319.
Schumacher, J. W., McCann, M. K., Maximov, K. J., & Fitzpatrick, D. (2021). Selective enhancement of neural coding in V1 underlies fine-discrimination learning in tree shrew. Current Biology, 32(15), 3245-3260.
2020
Keller, A. J., Dipoppa, M., Roth, M. M., Caudill, M. S., Ingrosso, A., Miller, K. D., & Scanziani, M. (2020). A disinhibitory circuit for contextual modulation in primary visual cortex. Neuron, 108(6), 1181-1193.
Lu, J., Behbahani, A. H., Hamburg, L., Westeinde, E. A., Dawson, P. M., Lyu, C., ... & Wilson, R. I. (2020). Transforming representations of movement from body-to world-centric space. Nature, 601(7891), 98-104.
Scholl, B., Thomas, C. I., Ryan, M. A., Kamasawa, N., & Fitzpatrick, D. (2020). Cortical response selectivity derives from strength in numbers of synapses. Nature, 590(7844), 111-114.
Robinson, N. T., Descamps, L. A., Russell, L. E., Buchholz, M. O., Bicknell, B. A., Antonov, G. K., ... & Häusser, M. (2020). Targeted activation of hippocampal place cells drives memory-guided spatial behavior. Cell, 183(6), 1586-1599.
Fan, J. L., Rivera, J. A., Sun, W., Peterson, J., Haeberle, H., Rubin, S., & Ji, N. (2020). High-speed volumetric two-photon fluorescence imaging of neurovascular dynamics. Nature Communications, 11(1), 6020.
Okubo, T. S., Patella, P., D’Alessandro, I., & Wilson, R. I. (2020). A neural network for wind-guided compass navigation. Neuron, 107(5), 924-940.
Mazzarda, F., D'Elia, A., Massari, R., De Ninno, A., Bertani, F. R., Businaro, L., ... & Mammano, F. (2020). Organ-on-chip model shows that ATP release through connexin hemichannels drives spontaneous Ca 2+ signaling in non-sensory cells of the greater epithelial ridge in the developing cochlea. Lab on a Chip, 20(16), 3011-3023.
Bowen, Z., Winkowski, D. E., & Kanold, P. O. (2020). Functional organization of mouse primary auditory cortex in adult C57BL/6 and F1 (CBAxC57) mice. Scientific Reports, 10(1), 10905.
Feng, K., Sen, R., Minegishi, R., Dübbert, M., Bockemühl, T., Büschges, A., & Dickson, B. J. (2020). Distributed control of motor circuits for backward walking in Drosophila. Nature Communications, 11(1), 6166.
Keller, A. J., Roth, M. M., & Scanziani, M. (2020). Feedback generates a second receptive field in neurons of the visual cortex. Nature, 582(7813), 545-549.
Eschbach, C., Fushiki, A., Winding, M., Afonso, B., Andrade, I. V., Cocanougher, B. T., ... & Zlatic, M. (2020). Circuits for integrating learnt and innate valences in the fly brain. bioRxiv, 058339.
Montgomery, M. K., Kim, S. H., Dovas, A., Zhao, H. T., Goldberg, A. R., Xu, W., ... & Hillman, E. M. (2020). Glioma-induced alterations in neuronal activity and neurovascular coupling during disease progression. Cell Reports, 31(2), 107500.
Shiozaki, H. M., Ohta, K., & Kazama, H. (2020). A multi-regional network encoding heading and steering maneuvers in Drosophila. Neuron, 106(1), 126-141.
Mestre, H., Du, T., Sweeney, A. M., Liu, G., Samson, A. J., Peng, W., ... & Nedergaard, M. (2020). Cerebrospinal fluid influx drives acute ischemic tissue swelling. Science, 367(6483), eaax7171.
Jeng, J. Y., Ceriani, F., Hendry, A., Johnson, S. L., Yen, P., Simmons, D. D., ... & Marcotti, W. (2020). Hair cell maturation is differentially regulated along the tonotopic axis of the mammalian cochlea. The Journal of Physiology, 598(1), 151-170.
Kovacs-Oller, T., Ivanova, E., Bianchimano, P., & Sagdullaev, B. T. (2020). The pericyte connectome: spatial precision of neurovascular coupling is driven by selective connectivity maps of pericytes and endothelial cells and is disrupted in diabetes. Cell Discovery, 6(1), 39.
Oe, Y., Wang, X., Patriarchi, T., Konno, A., Ozawa, K., Yahagi, K., ... & Hirase, H. (2020). Distinct temporal integration of noradrenaline signaling by astrocytic second messengers during vigilance. Nature Communications, 11(1), 471.
2019
Romero, S., Hight, A. E., Clayton, K. K., Resnik, J., Williamson, R. S., Hancock, K. E., & Polley, D. B. (2019). Cellular and widefield imaging of sound frequency organization in primary and higher order fields of the mouse auditory cortex. Cerebral Cortex, 30(3), 1603-1622.
Nardin, C., Peres, C., Mazzarda, F., Ziraldo, G., Salvatore, A. M., & Mammano, F. (2019). Photosensitizer activation drives apoptosis by interorganellar Ca2+ transfer and superoxide production in bystander cancer cells. Cells, 8(10), 1175.
Scholl, B., Wilson, D. E., Jaepel, J., & Fitzpatrick, D. (2019). Functional logic of layer 2/3 inhibitory connectivity in the ferret visual cortex. Neuron, 104(3), 451-457.
Ceriani, F., Johnson, S. L., Sedlacek, M., Hendry, A., Kachar, B., Marcotti, W., & Mammano, F. (2019). Dynamic coupling of cochlear inner hair cell intrinsic Ca2+ action potentials to Ca2+ signaling of non-sensory cells. bioRxiv, 731851.
Brawek, B and Garaschuk O. (2019). Single-cell electroporation for measuring in vivo calcium dynamics in microglia. Microglia Methods and Protocols, 231-241.
Brawek, B., Olmedillas del Moral, M., & Garaschuk, O. (2019). In vivo visualization of microglia using tomato lectin. Microglia Methods and Protocols, 165-175.
Liang, Y. & Garaschuk O. (2019). Labeling microglia with genetically encoded calcium indicators. Microglia Methods and Protocols, 243–265.
Royzen, F., Williams, S., Fernandez, F. R., & White, J. A. (2019). Balanced synaptic currents underlie low-frequency oscillations in the subiculum. Hippocampus, 29(12), 1178-1189.
Rathore, A. P. S., Mantri, C. K., Aman, S. A. B., Syenina, A., Ooi, J., Jagaraj, C. J., ... & St. John, A. L. (2019). Dengue virus-elicited tryptase induces endothelial permeability and shock. Journal of Clinical Investiagtion, 129(10), 4180-4193.
Stringer, C., Pachitariu, M., Steinmetz, N., Carandini, M., & Harris, K. D. (2019). High-dimensional geometry of population responses in visual cortex. Nature, 571(7765), 361–365.
Ziraldo, G., Buratto, D., Kuang, Y., Xu, L., Carrer, A., Nardin, C., ... & Mammano, F. (2019). A human-derived monoclonal antibody targeting extracellular connexin domain selectively modulates hemichannel function. Frontiers in Physiology, 10, 392.
Díaz-García, C. M., Lahmann, C., Martínez-François, J. R., Li, B., Koveal, D., Nathwani N., ... & Yellen, G. (2019). Quantitative in vivo imaging of neuronal glucose concentrations with a genetically encoded fluorescence lifetime sensor. Journal of Neuroscience Research, 97(8), 946–960.
Philip, V., Newton, D., Oh, H., Collins, S., Bercik, P., & Sibille, E. (2019). The Effect of Gut Microbiota on Glutamatergic/GABAergic Gene Expression in Adult Mice. Biological Psychiatry, 85, S127–S128.
Bowen, Z., Winkowski, D. E., Seshadri, S., Plenz, D., & Kanold, P. O. (2019). Neuronal avalanches in input and associative layers of auditory cortex. Frontiers in Systems Neuroscience, 13, 45.
Burgold, J., Schulz-Trieglaff, E. K., Voelkl, K., Gutiérrez-Ángel, S., Bader, J. M., Hosp, F., ... & Dudanova, I. (2019). Cortical circuit alterations precede motor impairments in Huntington’s disease mice. Scientific Reports, 9(1), 6634.
Matovic, S., Ichiyama, A., Igarashi, H., Salter, E. W., Wang, X. F., Henry, M., ... & Inoue, W. (2019). Stress-induced neuronal hypertrophy decreases the intrinsic excitability in stress habituation. bioRxiv, 593665.
Weissenberger, Y., King, A. J., & Dahmen, J. C. (2019). Decoding mouse behavior to explain single-trial decisions and their relationship with neural activity. bioRxiv, 567479.
Ceriani, F., Hendry, A., Jeng, J. Y., Johnson, S. L., Stephani, F., Olt, J., ... & Marcotti, W. (2019). Coordinated calcium signalling in cochlear sensory and non-sensory cells refines afferent innervation of outer hair cells. The EMBO Journal, 38(9), e99839.
Lee, K. S., Vandemark, K., Mezey, D., Shultz, N., & Fitzpatrick, D. (2019). Functional synaptic architecture of callosal inputs in mouse primary visual cortex. Neuron, 101(3), 421-428.
2018
Marvin, J. S., Scholl, B., Wilson, D. E., Podgorski, K., Kazemipour, A., Müller, J. A., ... & Looger, L. L. (2018). Stability, affinity, and chromatic variants of the glutamate sensor iGluSnFR. Nature Methods, 15(11), 936–939.
Moeyaert, B., Holt, G., Madangopal, R., Perez-Alvarez, A., Fearey, B. C., Trojanowski, N. F., ... & Schreiter, E. R. (2018). Improved methods for marking active neuron populations. Nature Communications, 9(1), 440.
Chen, C. L., Hermans, L., Viswanathan, M. C., Fortun, D., Aymanns, F., Unser, M., ... & Ramdya, P. (2018). Imaging neural activity in the ventral nerve cord of behaving adult Drosophila. Nature Communications, 9(1), 4390.
Corns, L. F., Johnson, S. L., Roberts, T., Ranatunga, K. M., Hendry, A., Ceriani, F., ... & Marcotti, W. (2018). Mechanotransduction is required for establishing and maintaining mature inner hair cells and regulating efferent innervation. Nature Communications, 9(1), 4015.
Saleem, A. B., Diamanti, E. M., Fournier, J., Harris, K. D., & Carandini, M. (2018). Coherent encoding of subjective spatial position in visual cortex and hippocampus. Nature, 562(7725), 124-127.
Dipoppa, M., Ranson, A., Krumin, M., Pachitariu, M., Carandini, M., & Harris, K. D. (2018). Vision and locomotion shape the interactions between neuron types in mouse visual cortex. Neuron, 98(3), 602-615.
Gillet, S. N., Kato, H. K., Justen, M. A., Lai, M., & Isaacson, J. S. (2018). Fear learning regulates cortical sensory representations by suppressing habituation. Frontiers in Neural Circuits, 11, 112.
2017
Scholl, B., Wilson, D. E., & Fitzpatrick, D. (2017). Local order within global disorder: synaptic architecture of visual space. Neuron, 96(5), 1127-1138.
Klapoetke, N. C., Nern, A., Peek, M. Y., Rogers, E. M., Breads, P., Rubin, G. M., ... & Card, G. M. (2017). Ultra-selective looming detection from radial motion opponency. Nature Neuroscience, 551(7679), 237-241.
Kato, H. K., Asinof, S. K., & Isaacson, J. S. (2017). Network-level control of frequency tuning in auditory cortex. Neuron, 95(2), 412-423.
Lu, R., Sun, W., Liang, Y., Kerlin, A., Bierfeld, J., Seelig, J.D., W... & Ji, N. (2017). Video-rate volumetric functional imaging of the brain at synaptic resolution. Nature Neuroscience, 20(4), 620-628.
2016
Mongeon, R., Venkatachalam, V., & Yellen, G. (2016). Cytosolic NADH-NAD+ redox visualized in brain slices by two-photon fluorescence lifetime biosensor imaging.. Antioxid Redox Signal, 25(10), 553-563.
Pachitariu, M., Stringer, C., Schröder, S., Dipoppa, M., Rossi, L. F., Carandini, M., & Harris, K. D. (2016). Suite2p: beyond 10,000 neurons with standard two-photon microscopy. bioRxiv, 061507.
Rose, T., Jaepel, J., Hübener, M., & Bonhoeffer, T. (2016). Cell-specific restoration of stimulus preference after monocular deprivation in the visual cortex. Science, 352(6291), 1319–1322.
Strobl, M. J., Freeman, D., Patel, J., Poulsen, R., Wendler, C. C., Rivkees, S. A., & Coleman, J. E. (2016). Opposing effects of maternal hypo-and hyperthyroidism on the stability of thalamocortical synapses in the visual cortex of adult offspring.. Cerebral Cortex, 27(5), 3015-3027.
Lee, K. S., Huang, X., & Fitzpatrick, D. (2016). Topology of ON and OFF inputs in visual cortex enables an invariant columnar architecture. Nature, 533(7601), 90-94.
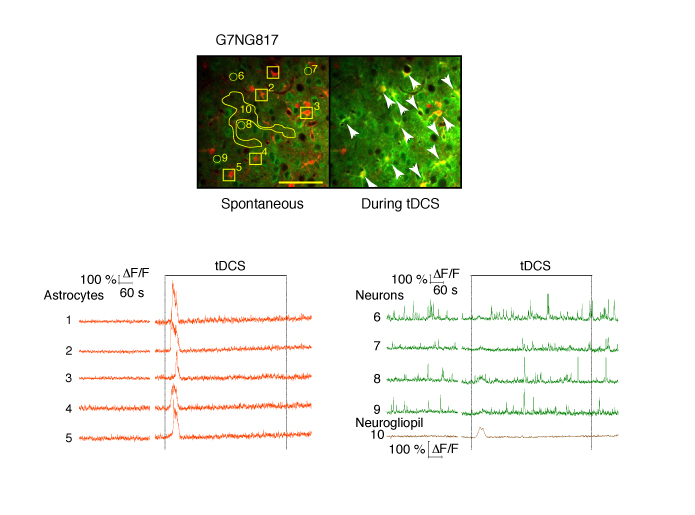
Monai, H., Ohkura, M., Tanaka, M., Oe, Y., Konno, A., Hirai, H., ... & Hirase, H. (2016). Calcium imaginq reveals glial involvement in transcranial direct current stimulation-induced plasticity in mouse brain. Nature Communications, 7(1), 11100.
Ganmor, E., Krumin, M., Rossi, L. F., Carandini, M., & Simoncelli, E. P. (2016). Direct estimation of firing rates from calcium imaging data. arXiv, 1601.00364.
2015
Roth, M. M., Dahmen, J.C., Muir, D. R., Imhof, F., Martini, F. J., & Hofer, S. B. (2015). Thalamic nuclei convey diverse contextual information to layer 1 of visual cortex. Nature Neuroscience, 19(2), 299-307.
Barnstedt, O., Keating, P., Weissenberger, Y., King, A. J., & Dahmen, J. C. (2015). Functional microarchitecture of the mouse dorsal inferior colliculus revealed through in vivo two-photon calcium imaging.. Journal of Neuroscience, 35(31), 10927-10939.
Chen, S. X., Kim, A. N., Peters, A. J., & Komiyama, T. (2015). Subtype-specific plasticity of inhibitory circuits in motor cortex during motor learning. Nature Neuroscience, 18(8), 1109-1115.
Jia, Y., Zhang, S., Miao, L., Wang, J., Jin, Z., Gu, B., ... & Li, Z. (2015). Activation of platelet protease-activated receptor-1 induces epithelial-mesenchymal transition and chemotaxis of colon cancer cell line SW620. Oncology Reports, 33(6), 2681-2688.
Lu, W., Tang, Y., Zhang, Z., Zhang, X., Yao, Y., Fu, C., ... & Ma, G. (2015). Inhibiting the mobilization of Ly6Chigh monocytes after acute myocardial infarction enhances the efficiency of mesenchymal stromal cell transplantation and curbs myocardial remodeling.. American Journal of Translational Research, 7(3), 587-597.
Boyd, A. M., Kato, H. K., Komiyama, T., & Isaacson, J. S. (2015). Broadcasting of cortical activity to the olfactory bulb. Cell Reports, 10(7), 1032-1039.
Cossell, L., Iacaruso, M. F., Muir, D. R., Houlton, R., Sader, E. N., Ko, H., H... & Mrsic-Flogel, T. D. (2015). Functional organization of excitatory synaptic strength in primary visual cortex. Nature, 518(7539), 399-403.
2014
Partridge, J. G., Lewin, A. E., Yasko, J. R., & Vicini, S. (2014). Contrasting actions of group I metabotropic glutamate receptors in distinct mouse striatal neurones. Journal of Physiology, 592(13), 2721-2733.
Peters, A. J., Chen, S, X., Komiyama, T. (2014). Emergence of reproducible spatiotemporal activity during motor learning. Nature, 510(7504), 263-267.
Ehmke, T., Nitzsche, T. H., Knebl, A., & Heisterkamp, A. (2014). Molecular orientation sensitive second harmonic microscopy by radially and azimuthally polarized light. Biomedical Optics Express, 5(7), 2231-46.
Liu, J., Wu, N., Ma, L., Liu, M., Liu, G., Zhang, Y., & Lin, X. (2014). Oleanolic acid suppresses aerobic glycolysis in cancer cells by switching pyruvate kinase type M isoforms. PLoS One, 9(3), e91606.
Palmer, L. M., Shai, A. S., Reeve, J.E., Anderson, H. L., Paulsen, O., & Larkum, M. E. (2014). NMDA spikes enhance action potential generation during sensory input. Nature Neuroscience, 17(3), 383-390.
Cai, F., Yu, J., Qian, J., Wang, Y., Chen, Z., Huang, J., ... & He, S. (2014). Use of tunable second-harmonic signal from KNbO3 nanoneedles to find optimal wavelength for deep-tissue imaging. Laser & Photonics Reviews, 8(6), 865-874.
2013
Kato, H. K., Gillet, S. N., Peters, A. J., Isaacson, J. S., & Komiyama, T. (2013). Parvalbumin-expressing interneurons linearly control olfactory bulb output. Neuron, 80(5), 1218-1231.
Takata, N., Nagai, T., Ozawa, K., Oe, Y., Mikoshiba, K., & Hirase, H. (2013). Cerebral blood flow modulation by Basal forebrain or whisker stimulation can occur independently of large cytosolic Ca2+ signaling in astrocytes. PLoS One, 8(6), e66525.
![]()
ThorImage®LSのソースコードは、Bergamo®、Cerna® 対応共焦点顕微鏡、Veneto®または共焦点顕微鏡をお持ちのお客様にご提供可能です。当社にメールにてご連絡ください。
ThorImage®LSソフトウェア
ThorImageLSは、当社の顕微鏡ならびに補助的な外付けハードウェアを制御するオープンソースの画像取得プログラムです。切片の多光子Zスタックからin vivoの同時光刺激やイメージングまで、ThorImageLSはそれぞれのニーズに合わせて組み込まれたモジュール式のワークスペースをご提供しております。そのワークフロー指向のインターフェイスは、単一画像、Zスタック、タイムシリーズ、そしてストリーミング画像の取得、可視化ならびに解析をサポートします。ThorImageLSのデータ取得ならびに解析の様子が右下の動画でご覧いただけます。
ThorImageLSは当社の顕微鏡をお買い求めいただくと付属しています。またオープンソースのため、ソフトウェア機能や性能の完全カスタマイズが可能です。ThorImageLSには当社のカスタマーサポートならびに定期的なソフトウェア更新サービスが付帯しており、常にイメージングの需要に合うよう心がけております。
詳細については製品紹介ページをご覧ください。
高機能ソフトウェア
- カスタマイズ可能な複数の表示欄を持つワークスペース
- ハードウェア入力とタイミング同期した画像取得
- ライブ画像の補正と関心領域の解析
- ガルバノ-ガルバノならびにガルバノ-レゾナント走査の領域・形状の個別設定
- タイリングによる高分解能広域イメージング
- 高速組織深部スキャンに適した1次、2次Z軸の個別制御
- スクリプトを使用した自動画像キャプチャ
- ImageJ Macrosに対応
- ワークステーション共有時にもマルチユーザの設定を保存
- 検出チャンネル毎に異なるカラー表示で簡単なビジュアル解析
実験でのシームレスな組み込み
- 空間変調モジュールを使用した同時多点光刺激とイメージング
- PFM450Eまたはサードパーティの対物レンズスキャナを使用した高速Zスタック取得
- 電気生理学のための信号制御
- Tiberius®レーザまたはCoherent社のChameleonレーザを使用した波長切り替え
- ポッケルスセルによる関心領域のマスキング
- 深度に応じたパワーランプ制御でダメージを最小に抑制、Signal to Noise 比を最大化
新機能: Version 4.3(クリックしてご覧ください。)
お持ちの顕微鏡に対応する最新のThorImageLSについては当社までお問い合わせください。ThorImageLS 4.xは、バージョン3. x 、2.xならびに1.xに新規機能を大幅に追加しており、旧モデルの顕微鏡に対応できない場合があります。当社では旧モデルをお持ちのお客様のために旧バージョンのソフトウェアのサポートを継続しております。旧バージョンの機能についてはこちらをご覧ください。
- Added Support for the Toptica iChrome CLE-50 Laser
- Added Support for 3P Imaging
- Added Support for the CS126MU, CS165MU, and CC505MU Monochrome Cameras
- Added Support for a Mini-Circuits® Switch Box
- Added Support for PMT3100R (Included in Some Bergamo II Multiphoton Imaging Systems)
- Added Configurable Channel View Layout (Horizontal and Vertical)
- Added Improved Scan Path Realignment and Added Ability to Save Multiple Reference Images for Multiple Targets
- Allows for Simplified Relocation to Same Target Day to Day
- Added New Features for SLM Operation
- Added 3D Mode Toggle
- Added Z Offset
- Added Ability to Export Patterns
- Added Set Zero% and Delete All Buttons
- Added Pattern Center to Display as "0" Point
- Added SLM Control Panel Advanced Mode Enhancement
- Added New Optional Delay Between Epochs
- Added SLM Control Panel Import/Export Enhancement
- Ability to Export Table of SLM Patterns
- Added New SLM Settings
- Added to ThorSLMSettings.xml and Application Settings
- Added Ability to Offset the Z Position of Pattern Points in New 3D Mode
- Enhanced ORCA Fusion Features
- Updated Exposure Calculation for Master Pulse Mode
- Added Option to Enable Water Cooling Control
- Added Improved Performance When Switching Modalities
- Enhanced Two-Way Scanning
- Two-Way Scanning is Now Allowed Up to a Pixel Density of 4096 x 4096 When Only One Channel is Selected
- Only Selectable by Dragging the Slider Bar to the Desired Pixel Density in One-Way Before Switching to Two-Way
- Added Camera Frame Rate Control
- Added UI Control of Two Blue Mini-Circuits® Switch Boxes
- Added 3D SLM
- Added Continuous Preview and Enhanced Orthogonal Views for Z-Stacks
- Added Improved Laser Safety Control for Digital Switches When Switches Are Configured for Laser Switching
- Added Control for Stimulus Shutter Operation When Using Stimulus Capture Mode
- Added Camera Frame Rate Control
- Added a New Section to Control the Frame Rate for CMOS Cameras with this Functionality
- Added Image-Based Autofocus
- Added Ability to Find the Optimal Focus Point of the Sample Based on Image Contrast
- Added Automatic Version Update Checker
- When Connected to Internet a Version Update Check Will Occur as the Splash Screen Loads When ThorImageLS Is Started Up
- Added Signal Generator Analog Mode
- Allows Custom Control of Analog Modulation
- Added New Continuous Button for Repeated Preview
- Allows for Fast Location Sample in XZ and YZ Line Scan Mode
- Added Enhanced IPC Communication
- Added IPC Command to Load an Experiment or a Template
- Added IPC Commands to Move X, Y, Z, and Secondary Z Stages
- Added IPC Commands that Get Sent from ThorImageLS Every Time a File Is Saved During T Series Experiments
ThorImage®LSの特長
| Laser Scanning | |||
|---|---|---|---|
| Scan Path Wavelength Range | 450 - 1100 nm, 680 - 1300 nm, or 800 - 1800 nm | ||
| Scan Paths | Resonant-Galvo-Galvo Scanner, Galvo-Resonant Scanners, Galvo-Galvo Scanners, or Spatial Light Modulator; Single or Dual Scan Paths | ||
| Scan Speed | 8 kHz Resonant-Galvo-Galvo or Galvo-Resonant | 2 fps at 4096 x 4096 Pixels 30 fps at 512 x 512 Pixels 400 fps at 512 x 32 Pixels | |
| 12 kHz or Galvo-Resonant | 4.4 fps at 2048 x 2048 Pixels 45 fps at 512 x 512 Pixels 600 fps at 512 x 32 Pixels | ||
| Galvo-Galvo | 3 fps at 512 x 512 Pixels 48 fps at 512 x 32 Pixels 70 fps at 32 x 32 Pixels Pixel Dwell Time: 0.4 to 20 µs | ||
| Galvo-Galvo Scan Modes | Imaging: Line, Polyline, Square, or Rectangle Non-Imaging: Circle, Ellipse, Polygon, or Point | ||
| Field of View | 20 mm Diagonal Square (Max) at the Intermediate Image Plane [12 mm Diagonal Square (Max) for 12 kHz Scanner] | ||
| Scan Zoom | 1X to 16X (Continuously Variable) | ||
| Scan Resolution | Up to 2048 x 2048 Pixels (Bi-Directional) [Up to 1168 x 1168 Pixels for 12 kHz Scanners] Up to 4096 x 4096 Pixels (Unidirectional) [Up to 2336 x 2336 Pixels for 12 kHz Scanners] | ||
| Compatible Objective Threadings | M34 x 1.0, M32 x 0.75, M25 x 0.75, and RMS | ||
| Multiphoton Signal Detection | ||
|---|---|---|
| Epi-Detection | Up to Four Ultrasensitive GaAsP PMTs, Cooled or Non-Cooled | |
| Forward-Direction Detection | Two Ultrasensitive GaAsP PMTs | |
| Maximum of Four PMTs Controlled by the Software at a Given Time | ||
| Collection Optics | 8°, 10°, or 14° Collection Angle (Angles Quoted When Using an Objective with a 20 mm Entrance Pupil) Easy-to-Exchange Emission Filters and Dichroic Mirrors | |
| Confocal Imaging | ||
|---|---|---|
| Motorized Pinhole Wheel with 16 Round Pinholes from Ø25 µm to Ø2 mm Two to Four Laser Lines (488 nm Standard; Other Options Range from 405 nm to 660 nm) Standard Multialkali or High-Sensitivity GaAsP PMTs Easy-to-Exchange Emission Filters and Dichroic Mirrors |
| Widefield Viewing | ||
|---|---|---|
| Manual or Motorized Switching Between Scanning and Widefield Modes Illumination Provided via LED or Liquid Light Guide C-Mount Threads for Scientific Cameras |
| Transmitted Light Imaging | ||
|---|---|---|
| Differential Interference Contrast (DIC) or Dodt Gradient Contrast Widefield or Laser Scanned Illumination Provided by Visible and/or NIR LEDs Compatible with Air or Oil Immersion Condensers |
| Three-Photon Imaging | ||
|---|---|---|
| Scan Optics for 800 - 1800 nm Range Achieve Reduced Background Scatter for Greater Sensitivity in Deep Tissue Imaging |
| Volume Imaging Using Bessel Beams | ||
|---|---|---|
| 3D Volumetric Functional Imaging at Video Frame Rates Enhanced Temporal Resolution for Studying Internal Systems at Cellular Lateral Resolution In Vivo |
| Translation | ||||
|---|---|---|---|---|
| Microscope Body Rotation (Rotating Bodies Only) | 0° to 90° or -45° to +45° Around Objective Focus 0.1° Encoder Resolution | |||
| Coarse Elevator Base Z (Rotating Bodies Only) | 5" (127 mm) Total Travel; 1 µm Encoder Resolution | |||
| Fine Microscope Body X and Y | 2" (50.8 mm) Total Travel; 0.5 µm Encoder Resolution | |||
| Fine Microscope Arm Z | 1" (25.4 mm) Total Travel; 0.1 µm Encoder Resolution | |||
| Fine Objective Z (Piezo Objective Scanner) | Open Loop: 600 µm ± 10% Travel Range; 1 nm Resolution Closed Loop: 450 µm Travel Range; 3 nm Resolution | |||
当社では、用途ごとのさまざまなご要望にお応えできるように、お客様のニーズに合わせたご提案を心掛けています。ご意見・ご要望、またご質問などございましたら当社までお気軽にご連絡ください。

Click to Enlarge
デモルーム例(中国オフィス)
デモルーム・オンラインデモのご案内
ソーラボの技術者は、世界9カ所のオフィスをベースにしており、お客様の実験用途に適したイメージングシステムをお選びいただくためのお手伝いをいたします。生物学のあらゆる課題解決に向けて研究を行うお客様のために、ニーズに合致し、かつ使いやすく、高い信頼性と対応力のあるシステムを提供いたします。
当社では、実際に当社顕微鏡システムなどを無償でお試しいただけるデモルームをご用意しています。オンラインデモも承ります。 デモルームやオンラインデモのご予約、お問い合わせは当社までご連絡ください。
カスタマーサポート(海外)
(クリックすると詳細がご覧いただけます)
Newton, New Jersey, USA
Thorlabs Headquarters
43 Sparta Avenue
Newton, NJ 07860
Customer Support
- Phone: (973) 300-3000
- E-mail: techsupport@thorlabs.com
Ely, United Kingdom
Thorlabs Ltd.
1 Saint Thomas Place, Ely
Ely CB7 4EX
Customer Support
- Phone: +44 (0)1353-654440
- E-mail: techsupport.uk@thorlabs.com
Bergkirchen, Germany
Thorlabs GmbH
Münchner Weg 1
85232 Bergkirchen
Customer Support
- Phone: +49 (0) 8131-5956-0
- E-mail: europe@thorlabs.com
Maisons-Laffitte, France
Thorlabs SAS
109, rue des Cotes
Maisons-Laffitte 78600
Customer Support
- Phone: +33 (0)970 440 844
- E-mail: techsupport.fr@thorlabs.com
São Carlos, SP, Brazil
Thorlabs Vendas de Fotônicos Ltda.
Rua Rosalino Bellini, 175
Jardim Santa Paula
São Carlos, SP, 13564-050
Customer Support
- Phone: +55-16-3413 7062
- E-mail: brasil@thorlabs.com
デモルームのご案内
(クリックすると詳細がご覧いただけます)
日本 (東京都練馬区)
ソーラボジャパン株式会社
東京都練馬区北町3-6-3
お問い合わせ
- Tel: 03-6915-7701
- Email: techsupport.jp@thorlabs.com
デモルーム常設顕微鏡システム *ほかのデモをご希望の場合もご相談ください。
Sterling, Virginia, USA
Thorlabs Imaging Systems HQ
108 Powers Court
Sterling, VA 20166
Customer Support
- Phone: (703) 651-1700
- E-mail: ImagingTechSupport@thorlabs.com
Demo Rooms
- Bergamo® II Series Multiphoton Microscopes
- Veneto® Inverted Microscopes
- Four-Channel Cerna®-Based Confocal Microscopes
- Cerna Birefringence Imaging Microscopes
- Multiphoton Mesoscope
- OCT Systems: Telesto® and Ganymede™
Lübeck, Germany
Thorlabs GmbH
Maria-Goeppert-Straße 9
23562 Lübeck
Customer Support
- Phone: +49 (0) 8131-5956-40840
- Email: oct@thorlabs.com
Demo Rooms
- Ganymede™ Series SD-OCT Systems
- Telesto® Series SD-OCT Systems
- Telesto® Series PS-OCT Systems
- Atria® Series SS-OCT Systems
- Vega™ Series SS-OCT Systems
Shanghai, China
Thorlabs China
Room A101, No. 100, Lane 2891, South Qilianshan Road
Shanghai 200331
Customer Support
- Phone: +86 (0)21-60561122
- Email: techsupport-cn@thorlabs.com
Demo Rooms
- Bergamo® II Series Multiphoton Microscopes
- Cerna Birefringence Imaging Microscopes
- OCT Systems: Telesto® and Ganymede™
| Posted Comments: | |
gaiqing Wang
(posted 2020-05-07 11:13:33.577) I am looking for a cheap way to do confocal imaging in vivo. Is this Bergamo II Series Multiphoton Microscope my best option? Can you send me a quote? YLohia
(posted 2020-05-07 09:45:11.0) Thank you for contacting Thorlabs. We will reach out to you directly to discuss your requirements. jfpena
(posted 2016-12-19 18:15:55.003) I am looking for a cheap way to do confocal imaging in vivo. Is this Bergamo II Series Multiphoton Microscope my best option? Can you send me a quote? tfrisch
(posted 2016-12-22 11:44:31.0) Hello, thank you for contacting Thorlabs. A member of our Imaging Team will reach out to you directly to discuss this system and your application. birech
(posted 2016-11-17 06:33:49.463) I asked for a price quote for this product, Bergamo II Series Multiphoton Microscopes three days ago. I am working at the University of Nairobi in Kenya and would wish to order one.
Regards,
Birech tfrisch
(posted 2016-11-17 06:56:23.0) Hello, thank you for contacting Thorlabs. I have forwarded this request to our Imaging Sales Team. I apologize for the delay. |
 Products Home
Products Home



















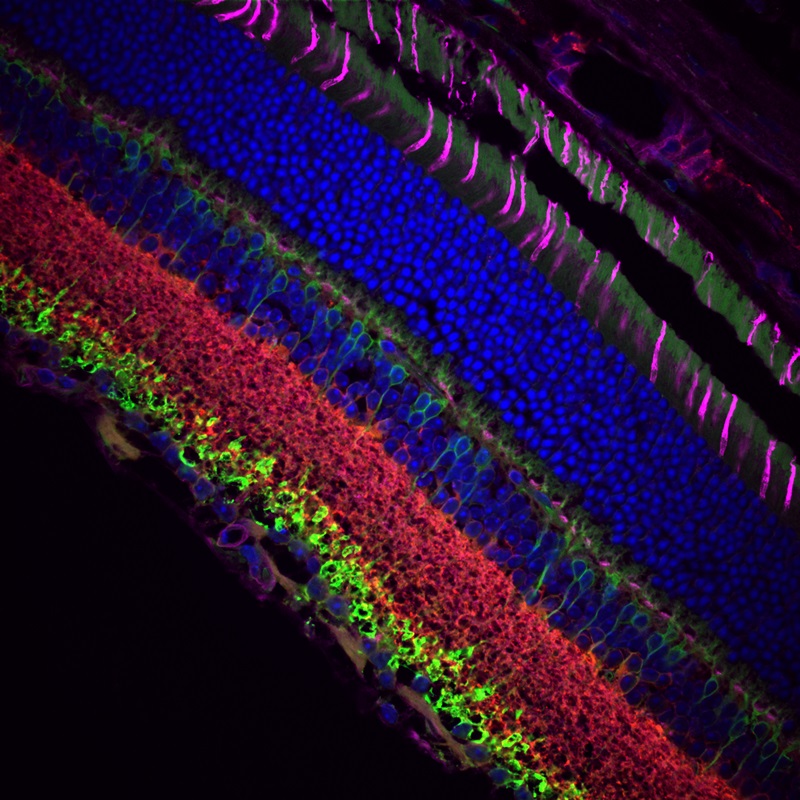
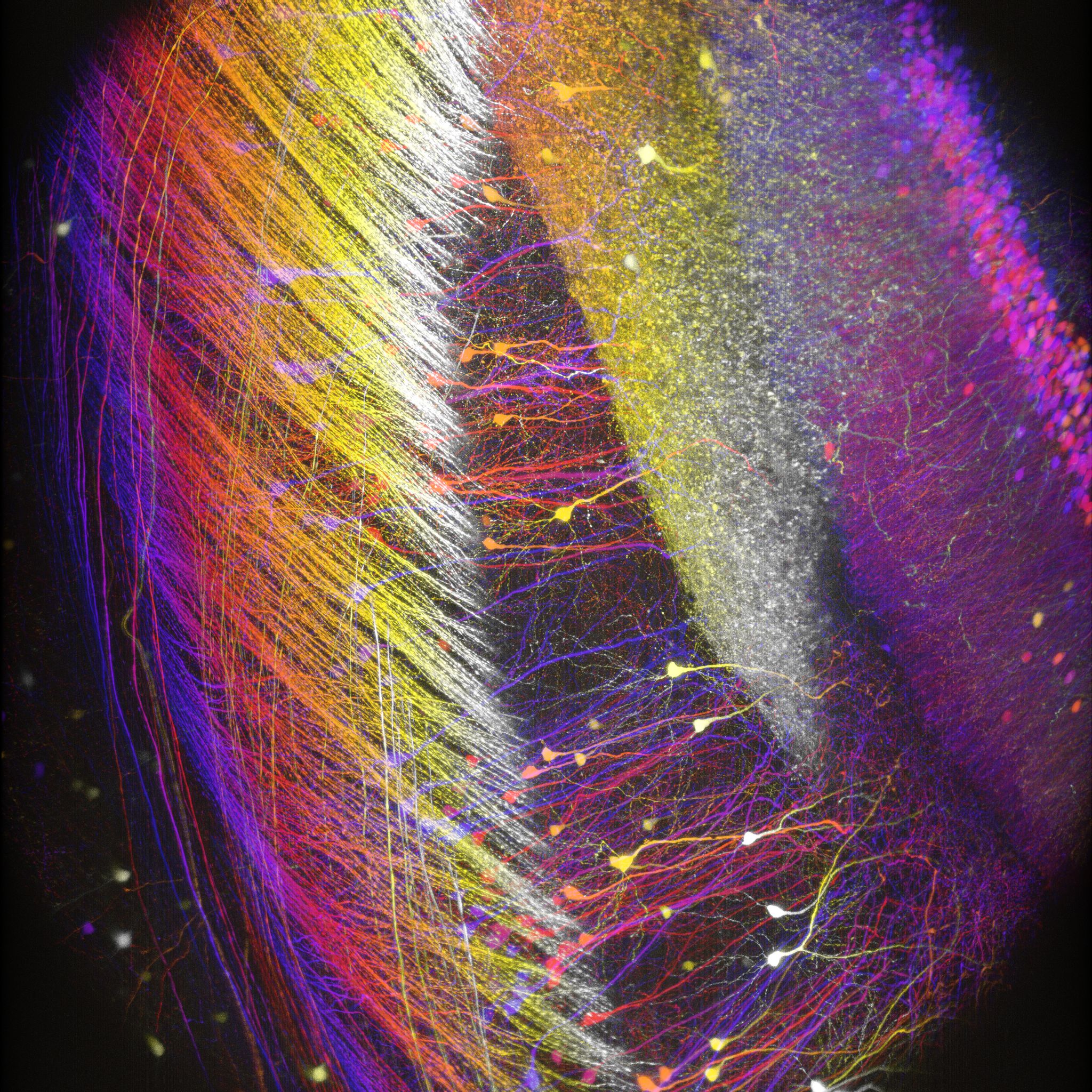
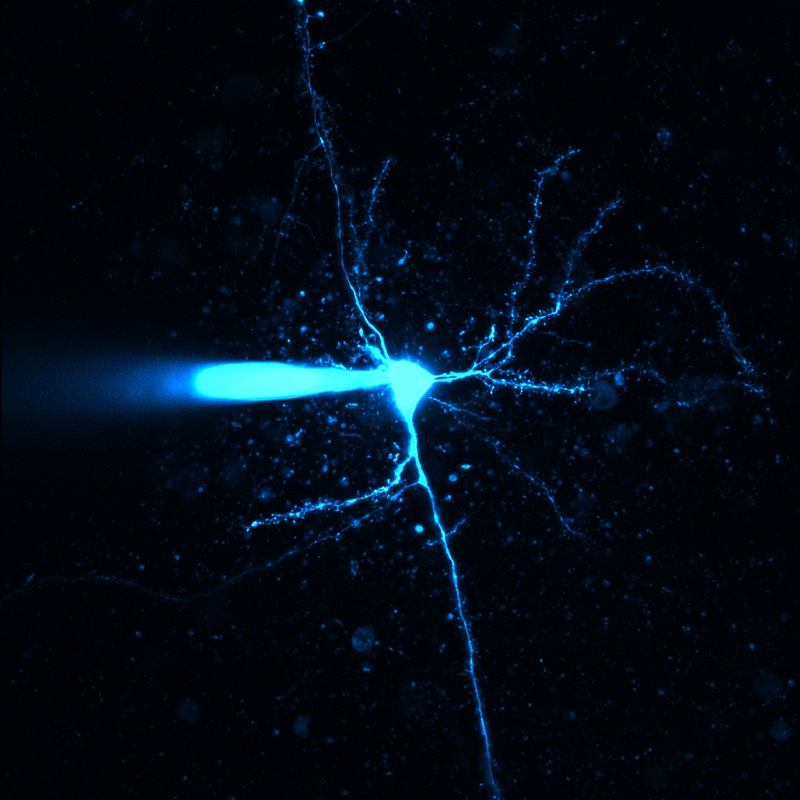
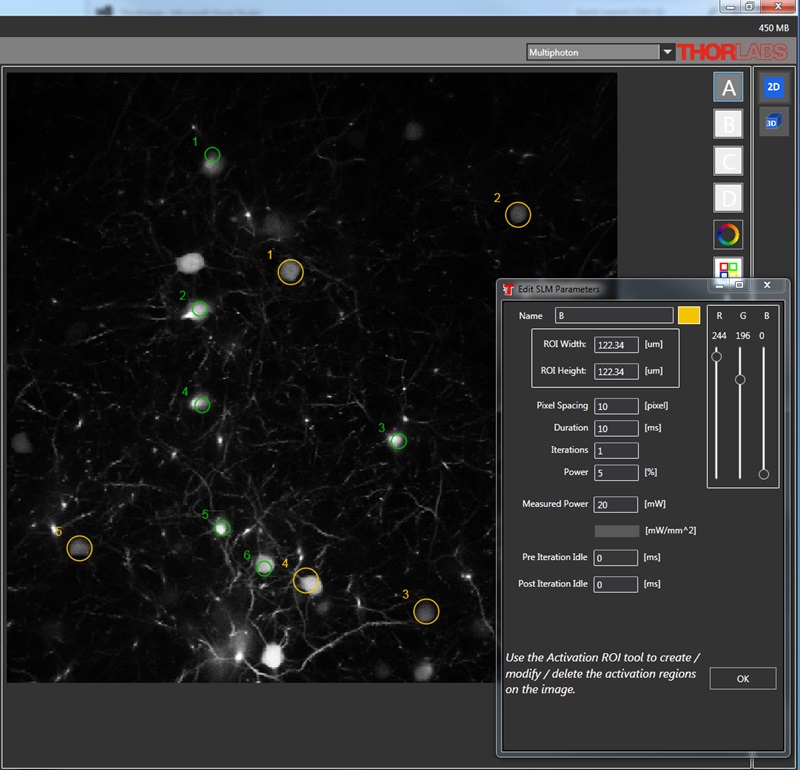
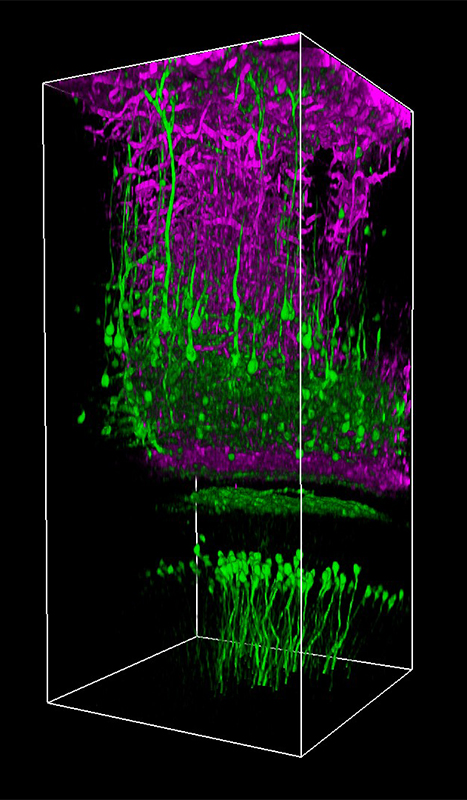
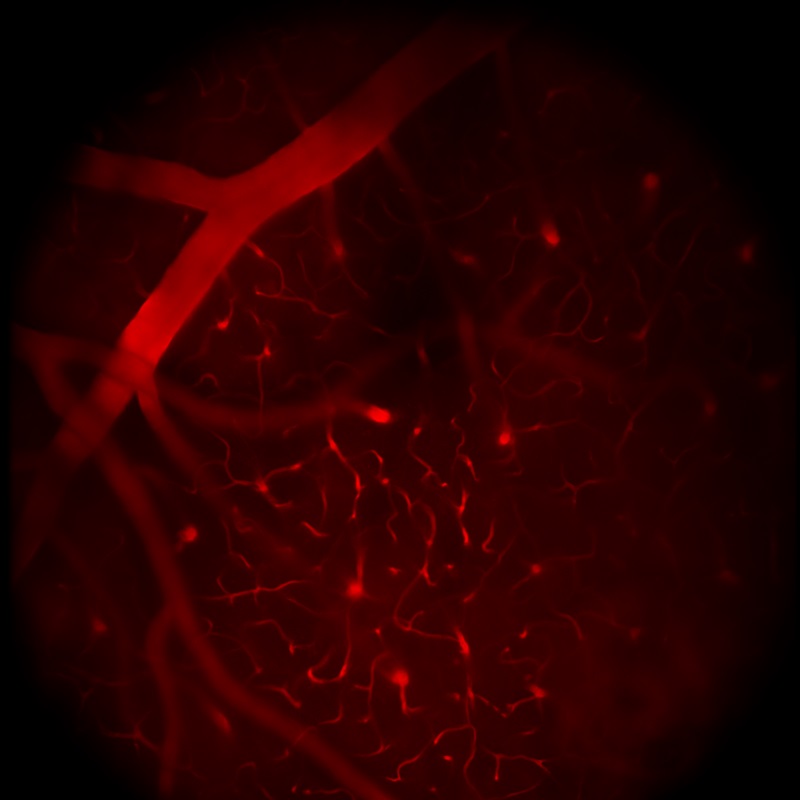
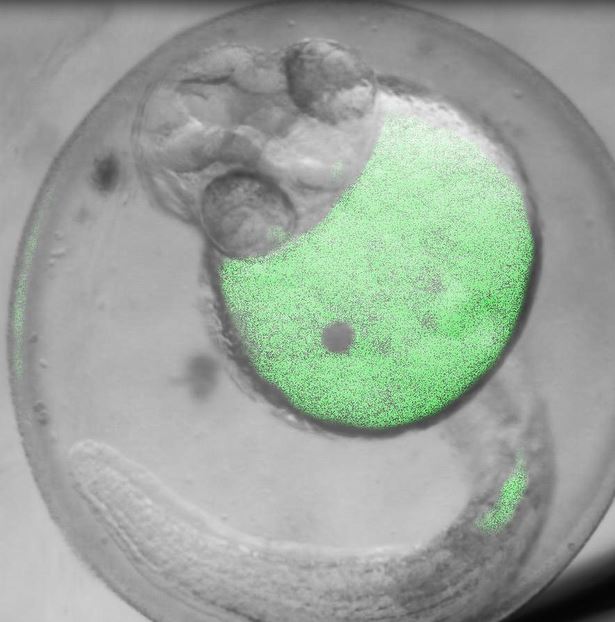
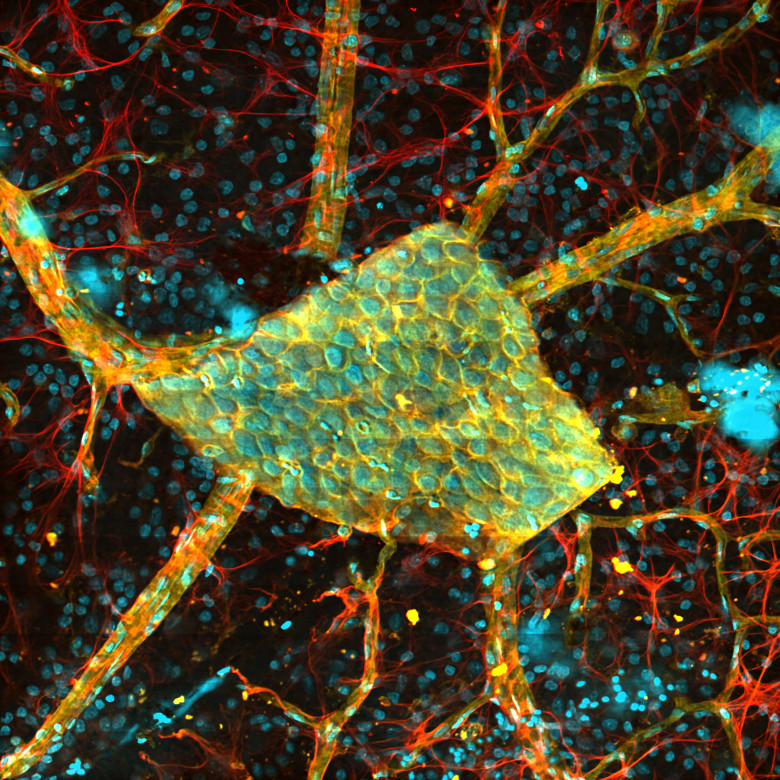
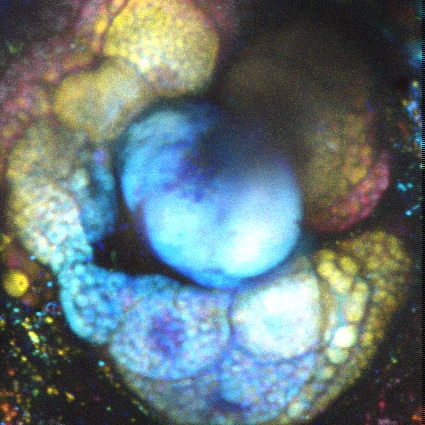
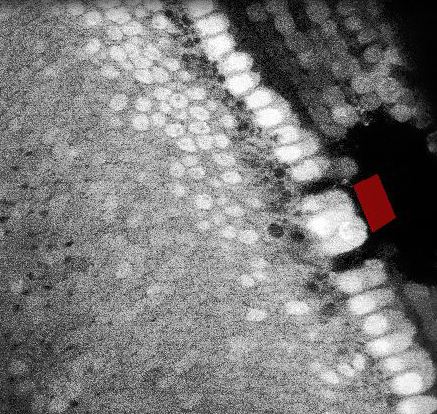
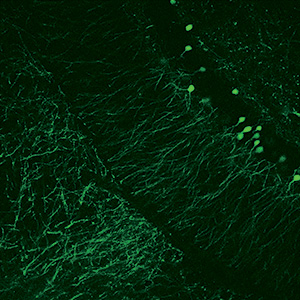


















 多光子顕微鏡:Bergamo® IIシリーズ
多光子顕微鏡:Bergamo® IIシリーズ